Professional Documents
Culture Documents
Metabolism.220.2012
Uploaded by
Cecilia NguyenOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metabolism.220.2012
Uploaded by
Cecilia NguyenCopyright:
Available Formats
133363003.
doc
BIO 220 6. Microbial Metabolism
TopicsOxidation/Reduction reactions Catabolic/Anabolic reactions Glycolysis Aerobic Respiration Anaerobic Respiration Fermentation Hydrocarbon Transformation Oxidation/Reduction Redox reactions These terms are discussed more thoroughly in chemistry class. However, as most cellular reactions are Redox reactions, it is difficult to understand Metabolism without a grasp of the meaning of these terms. Redox reactions involve the transfer of electrons during a chemical reaction. In this class we examine redox reactions in very simplified terms. If a molecule gains an electron during a chemical reaction it is reduced. As electrons are negatively charged, a molecule receiving an electron undergoes a charge reduction. If a molecule loses an electron during a chemical reaction it is oxidized. General redox reaction Ae- + B -> A + BeIn this reaction Ae- loses an electron (is oxidized) while B gains an electron (is reduced). 2 somewhat confusing terms-
133363003.doc
Oxidizing agent- a molecule which oxidizes another molecule by taking an electron away from that molecule. In the above reaction B acts as an oxidizing agent of Ae- by removing an electron from Ae-. Oxidizing agents become reduced. Reducing agent- a molecule which reduces another molecule by donating an electron to that molecule. In the above reaction Ae- acts as a reducing agent of B by donating an electron to B. Reducing agents become oxidized. Because an electron transfer requires a donor and a receptor, oxidation and reduction always go together. If we look at the reaction. C6H12O6 + O2 -> 6 CO2 + 6 H20 we see electrons are transferred from glucose to oxygen. Thus glucose is the reducing agent for it passes electrons to oxygen. Oxygen is the oxidizing agent for it accepts electrons from glucose. When viewing redox reactions we like to focus on the transfer of electrons. However hydrogen is sometimes added to the oxidizing agent as well to balance the negative charge of the electron. This is seen in the above reaction as oxygen accepts hydrogen (in addition to electrons) to form water. Redox Potential The change in energy from an electron donor to the electron acceptor is measured as the Redox Potential or Oxidation/Reduction coupling. Redox Potential is measured in volts. Figure 5.9 depicts an electron tower representing the redox potential between various electron donors and acceptors. Metabolism- the totality of chemical reactions within a cell. Comprises both Anabolic (synthesis) reactions and Catabolic (degradation) reactions. 2
133363003.doc
Heterotrophs extract energy from their environment by utilizing catabolic pathways. This includes the processes Glycolysis, Aerobic Respiration, Anaerobic Respiration and Fermentation. In these catabolic processes, complex molecules (such as glucose) are oxidized. The removed electrons are passed from one molecule to another in a series of Redox reactions. After each redox reaction the transferred electron contains slightly less energy. This step-wise removal of energy is ultimately used by the organism to generate ATP (Adenosine tri-phosphate). If the energy contained in the electron was liberated in a single step, the majority of the energy would be released as heat and thus not usable by the cell. The molecule which finally ends up with the electron is called the Terminal Electron Acceptor. Aerobic Respiration, Anaerobic Respiration and Fermentation all employ different Terminal Electron Acceptors. Role of NAD- Nicotinamide Adenine Dinucleotide in catabolism. NADH Cycling NAD cycles continuously between its oxidized form (NAD+) and its reduced form (NADH + H+). NADs ability to accept electrons then donate them to other molecules in specific biochemical pathways is critical to the cells ability to make ATP from organic molecules (glucose, amino acids, fatty acids). NAD Redox NAD+ + 2H + 2e- -> NADH + H+ NAD+ is the oxidized form of the molecule. NADH + H+ is the reduced form of the molecule. Short term and Long term sources of cellular energy Cells require a constant source of energy. ATP is a common source of short term energy. ATP hydrolysis (ATP-> ADP + Pi) is tremensdously exergonic (G =-34 kJ). The cell constantly recycles ADP to make ATP with the ration of ATP:ADP being approximately 1000:1). The cellular concentration of ATP is typically 2mM. CoEnzyme A, a molecule involved in the Citric Acid Cycle may serve as a short term supply of energy for anaerobes. Hydrolysis of the molecules thioester bonds yields sufficient free energy for other cellular processes. 3
133363003.doc
Long Term sources of energy include organic carbon molecules such as Polyglucose (sugar based), Poly--Hydroxybutyrate (lipid). Chemolithotrophs use inorganic metals such as elemental Sulfur as a source of long term energy.
Glycolysis Occurs in the cytoplasm 1 glucose broken down into 2 pyruvate 2 ATP are spent 4 ATP are generated (Net yield = 2 ATP) 2 NADH + H+ are generated Respiration In Respiration the 2 molecules of pyruvate generated by glycolysis are completely oxidized to CO2 in a series of biochemical reactions known as the Krebs Cycle (Citric Acid Cycle). During the oxidation of carbon compounds in the Krebs Cycle, NAD+ is reduced to NADH + H+ and a related compound FAD is reduced to FADH2. In addition to energy storing compounds NADH + H+ and FADH2, the Kebs Cycle also generates GTP (guanosine triphosphate). GTP is converted to ATP via substrate level phosphorylation. Overall each cycle of the Krebs Cycle generates4 molecules of NADH + H+ 1 molecule FADH2 1 molecule of GTP (that can be converted later to ATP)
NADH + H+ (and FADH2) participate in further redox reactions, donating electrons to a chain of proteins known as the Electron Transport Chain (ETC). The passage of electrons along the ETC is chemiosmotically linked to the generation of ATP.
133363003.doc
Free Energy and the ETC In terms of free energy relative to the terminal electron acceptor, each protein in the ETC has slightly less free energy than the protein preceding it. Each component of ETC has a higher affinity for electrons than the preceding component, with the terminal electron acceptor having the highest affinity of all. The decreasing free energy and increasing electron affinity of the ETC components keep the electrons moving unidirectionally down the ETC. Energy is released with each step in the ETC. This energy is not released as heat but transferred to another form of energy called a Proton Motive Force. See Figures 5.19 and 5.20.
Chemiosmosis in Prokaryotes Electrons are passed from NADH (and a related molecule FADH2) to a series of enzyme complexes (ETC) in the Plasma Membrane. The electrons are then passed through these enzymes in a series of oxidation/reduction reactions. As this occurs a H+ gradient is generated outside the plasma membrane. Such a gradient contains energy called a proton motive force. The proton motive force is utilized to drive the reaction ADP + Pi -> ATP (Oxidative Phosphorylation), drive bacterial flagella and move molecules across the plasma membrane. Brock defines respiration where the terminal electron acceptor is an exogenous molecule. In Aerobic Respiration the terminal electron acceptor is oxygen. In Anaerobic Respiration the terminal electron acceptor is an inorganic compound other than oxygen (Nitrates, Sulphates are examples). Although, aerobic respiration generates more ATP, many of the enzymes in the Electron Transport Chains are the same for both aerobic and anaerobic respiration. In organisms which can utilize both pathways, gene expression of enzymes involved in anaerobic respiration occurs only in the absence of oxygen.
133363003.doc
Chemiosmotic Coupling of the proton motive force to ATP synthesis As H+ ions accumulate in the Periplasmic space, a pH gradient and electrical gradient develops. Taken together these create a form of energy called the proton motive force. H+ ions cannot cross the inner membrane freely, however they are able to pass through a protein complex called ATP Synthase. ATP Synthase is composed of many protein subunits and has 3 main parts: Rotor Rod Knob As H+ ions pass through the ATP Synthase the rotor spins in a clockwise direction which activated the catalytic site in the knob. The knob portion of the enzyme catalyzes the reactionADP + Pi -> ATP
See diagram on board.
ENERGY YIELD Aerobic RespirationTheoretical Yield1 NADH + H+ entering ETC -> 3 ATP 1 FADH2 -> 2 ATP Experimental Yield1 NADH + H+ -> 2.5-2.7 ATP Other uses for proton motive force besides ATP generation Maximum theoretical ATP yield from 1 glucoseGlycolysis2 ATP 6
133363003.doc
2 NADH + H+ Pyruvate -> Acetyl CoA 2 NADH + H+ Citric Acid Cycle 8 molecules of NADH + H+ 2 molecule FADH2 2 molecule of GTP Oxidative Phosphorylation (converts energy in NADH and FADH2 into ATP) 34 ATP 38 ATP are generated from 1 molecule of glucose.
FermentationFermentation involves the partial breakdown of sugars without the assistance of oxygen. Terminal electron acceptor is an organic (carbon based) molecule. Fermentation products can be acidic (lactic acid, formic acid) or neutral (acetoin, ethanol). Bacteria can be differentiated by the fermentation pathways they employ. The Energy Yield of fermentation is much less than obtained via respiration. Genes involved in fermentation pathways are expressed in anaerobic conditions.
You might also like
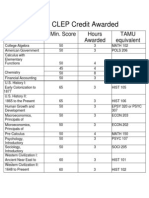
- Clep Exam Min. Score Hours Awarded Tamu EquivalentDocument1 pageClep Exam Min. Score Hours Awarded Tamu EquivalentCecilia NguyenNo ratings yet
- USMLE - AntibioticsDocument162 pagesUSMLE - AntibioticsCecilia Nguyen100% (1)
- Final Statistics Report-EY 06-DentalDocument1 pageFinal Statistics Report-EY 06-DentalCecilia NguyenNo ratings yet
- In Situ vs. Ex Situ: Aliphatic and Aromatic CompoundsDocument4 pagesIn Situ vs. Ex Situ: Aliphatic and Aromatic CompoundsCecilia NguyenNo ratings yet
- Bioremediation.220.F2012Document17 pagesBioremediation.220.F2012Cecilia NguyenNo ratings yet
- Bioremediation. 220.IODocument2 pagesBioremediation. 220.IOCecilia NguyenNo ratings yet
- Unfamiliar Symbols For SATDocument2 pagesUnfamiliar Symbols For SATCecilia NguyenNo ratings yet
- Critical Reading TutorialDocument65 pagesCritical Reading TutorialCecilia Nguyen100% (1)
- Microbiology Checklist (2011 Catalog) : Graduation RequirementsDocument1 pageMicrobiology Checklist (2011 Catalog) : Graduation RequirementsCecilia NguyenNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)