Professional Documents
Culture Documents
P10 18
Uploaded by
embargioOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
P10 18
Uploaded by
embargioCopyright:
Available Formats
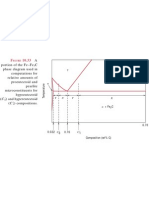
Refer to the C-rich portion of the Fe-Fe3C phase diagram.
(a) what is the eutectoid temperature and eutectoid composition? (b) what is the maximum solid solubility of C in ferrite? (c) what is the maximum solid solubility of C in austenite? (d) why is the solubility of C higher in austenite than in ferrite? (e) what is the maximum solubity of C in ferrite at T-200oC? (f) Consider a Fe-0.8wt%C alloy. For T = 1000oC and 700oC determine: what phases are present the composition of each phase how much of each phase is present a qualitative sketch of the microstructure (g) Suppose a Fe-0.8wt%C alloy is cooled slowly through the eutectoid temperature then: (i) held for a long time at 700oC; or (ii) quenched to room temperature. For each of these two cases, sketch the microstructure you would expect. Briefly justify your sketches.
You might also like
- Lubricating Oil, Internal Combustion EngineDocument21 pagesLubricating Oil, Internal Combustion EngineembargioNo ratings yet
- Friction & Wear Tester by Reichert M2Document4 pagesFriction & Wear Tester by Reichert M2embargioNo ratings yet
- P10 19Document1 pageP10 19embargioNo ratings yet
- TTT - DiagramsDocument13 pagesTTT - DiagramsembargioNo ratings yet
- Ch10 PhasesDocument11 pagesCh10 PhasesembargioNo ratings yet
- A Portion of The Fe-Fe C Phase Diagram Used in Computations For Relative Amounts of Proeutectoid and Pearlite Microconstituents For Hypoeutectoid (C ) and Hypereutectoid (C ) CompositionsDocument1 pageA Portion of The Fe-Fe C Phase Diagram Used in Computations For Relative Amounts of Proeutectoid and Pearlite Microconstituents For Hypoeutectoid (C ) and Hypereutectoid (C ) CompositionsembargioNo ratings yet
- Garrett Turbocharger CatalogDocument12 pagesGarrett Turbocharger Cataloghamiti100% (2)
- Iron-Carbon DiagramDocument11 pagesIron-Carbon DiagramrampradNo ratings yet
- Iron-Carbon DiagramDocument11 pagesIron-Carbon DiagramrampradNo ratings yet
- The iron-iron carbide (Fe-Fe C) phase diagram: α-ferrite austeniteDocument6 pagesThe iron-iron carbide (Fe-Fe C) phase diagram: α-ferrite austeniteembargioNo ratings yet
- Phase Diagram ExDocument23 pagesPhase Diagram ExTey KaijingNo ratings yet
- 3.14/3.40/22.71 Lecture Summary: Introduction To Steel Case StudyDocument7 pages3.14/3.40/22.71 Lecture Summary: Introduction To Steel Case StudyembargioNo ratings yet
- Iron - Iron Carbide Iron Iron Carbide Phase Diagram: Today's TopicsDocument11 pagesIron - Iron Carbide Iron Iron Carbide Phase Diagram: Today's TopicsembargioNo ratings yet
- Chapter 9: Phase Diagrams III: The Fe - Fe C Phase Diagram: Issues To Address..Document22 pagesChapter 9: Phase Diagrams III: The Fe - Fe C Phase Diagram: Issues To Address..embargioNo ratings yet
- A Portion of The Fe-Fe C Phase Diagram Used in Computations For Relative Amounts of Proeutectoid and Pearlite Microconstituents For Hypoeutectoid (C ) and Hypereutectoid (C ) CompositionsDocument1 pageA Portion of The Fe-Fe C Phase Diagram Used in Computations For Relative Amounts of Proeutectoid and Pearlite Microconstituents For Hypoeutectoid (C ) and Hypereutectoid (C ) CompositionsembargioNo ratings yet
- Ch10 PhasesDocument11 pagesCh10 PhasesembargioNo ratings yet
- Chapter 9: Phase Diagrams III: The Fe - Fe C Phase Diagram: Issues To Address..Document22 pagesChapter 9: Phase Diagrams III: The Fe - Fe C Phase Diagram: Issues To Address..embargioNo ratings yet
- The iron-iron carbide (Fe-Fe C) phase diagram: α-ferrite austeniteDocument6 pagesThe iron-iron carbide (Fe-Fe C) phase diagram: α-ferrite austeniteembargioNo ratings yet
- 8.fe - Fe3C Phase DiagramDocument27 pages8.fe - Fe3C Phase DiagramMhackSahuNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)