Professional Documents
Culture Documents
The Gallstone Story - Pathogenesis and Epidemiology
Uploaded by
Marlon WorthingtonCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Gallstone Story - Pathogenesis and Epidemiology
Uploaded by
Marlon WorthingtonCopyright:
Available Formats
DISEASES OF THE BILIARY TRACT, SERIES #4
R. M. Agrawal, M.D., Series Editor
The Gallstone Story: Pathogenesis and Epidemiology
Neil Bajwa
Rajinder Bajwa
Ambrish Ghumman
R. M. Agrawal
INTRODUCTION
allstone disease remains one of the most common medical conditions in the United States and in developed countries in general.14 Among biliary tract disease, it is the leading cause for inpatient admissions for gastrointestinal problems.5,6 In the United States in 2000 alone, there were 262,411 hospitalizations for cholecystitis and 778,632 outpatient visits.5 The average cost per each admission was $11,584.5 Between 19941998, an estimated 20.5 million American adults were diagnosed with cholelithiasis,1 which equates to approximately 15% of the American population. The most accurate and noninvasive method of predicting gallstone disease was achieved with the advent of the ultrasound, which has a sensitivity/specificity of greater than 95%. However, the true prevalence of the disease remains hard to
Neil Bajwa, MD; Rajinder Bajwa, MD; Ambrish Ghumman, MD; R.M. Agrawal, MD, FACG, AGAF, FASGE; Allegheny General Hospital, Drexel University College of Medicine, Division of Gastroenterology, Pittsburgh, PA.
derive as the majority of patients remain asymptomatic. Recent studies indicate that only 10%18% of patients with cholelithiasis develop symptoms such as nausea, vomiting and abdominal pain. This paper will review the pathogenesis and epidemiology of gallstone disease, focusing on both unmodifiable and modifiable risk factors.
PATHOGENESIS
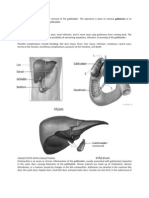
Gallstones are principally formed due to abnormal bile constituents (eg, cholesterol, phospholipids and bile salts). Cholesterol gallstone formation can be arbitrarily divided into 3 stages: cholesterol saturation, nucleation, and stone growth (Figure 1).7
Cholesterol Solubilization and Super Saturation
Cholesterol is virtually insoluble in aqueous solution and requires some vehicle to render it soluble in bile.7 This is overcome by secretion of phospholipids and bile salts along with cholesterol. The biliary lipids (lecithin, cholesterol, and bile salts) are secreted into the hepatic canaliculi by adenosine tri-phosphate
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
11
The Gallstone Story
DISEASES OF THE BILIARY TRACT, SERIES #4
CTP: Phosphocholine Cytidyly transferase Excretion and supersaturation Phosphatidyl choline (PC) (Lecithin)
HMG-Co A Reductase
Cholesterol 7-hydroxylase
Cholesterol (CH)
Bile salts (BS)
PL-CH vesicle
BS simple micelles
Unilamellar PC-CH-(BS) vesicles Fuse
Simple BS-CH micelles
Mixed BS-CH-PL micelles Nucleation Multilamellar PL-CH-(BS) vesicles
CH monohydrate crystals
Stone growth
Cholesterol gall stones
Figure 1. Pathogenesis of cholesterol gallstones.
(ATP)-dependent transport proteins.4 Soon after the excretion, cholesterol and lecithin combine to form metastable unilamellar vesicles, while the bile salts, after reaching a critical concentration, form simple micelles. The dynamic interaction of unilamellar vesicles with the simple micelles leads to the formation of mixed micelles during the passage through the biliary tract into the gallbladder.8,9 Studies have shown that vesicles are able to solublize more cholesterol than mixed micelles. The concentration of phospholipids and bile salts relative to cholesterol is thought to be the critical factor in determining the solublization and saturation of cholesterol in bile and thus making it more lithogenic if homeostasis is not maintained.
Nucleation of Cholesterol Crystals
Nucleation is the process by which cholesterol monohydrate crystals form and agglomerate.7 In supersaturated bile, the interaction between mixed micelles and vesicles lead to precipitation of cholesterol crystals. Video
12
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
enhanced microspic studies have shown that crystals appeared to originate from aggregated vesicles.7,10 This observation is explained by the fact that when the vesicles and mixed micelles interact in the gallbladder, the micelles remove phospholipids from the vesicles in preference to cholesterol, thus making vesicles rich in cholesterol.7 The remaining vesicles are relatively enriched in cholesterol and prone to nucleation. After nucleation, crystallization of the cholesterol can then occur eventually leading to the formation of macroscopic gallstones. Initially super saturation was thought to be the primary pathological event leading to the formation of gallstones, however with the advent of assays to determine the nucleation time and the observation of asymptomatic super saturation of gallstones in patients, it has become evident that nucleation of the cholesterol crystals plays a vital role in the overall development of gallstones. Nucleation time can be decreased by certain pro-nucleation factors such as gallbladder mucin, heatlabile glycoprotein, immunoglobulin, phospholipase C, and most likely, calcium.8 Factors which slow nucle-
The Gallstone Story
DISEASES OF THE BILIARY TRACT, SERIES #4
ation include apolipoprotein AI and AII and a 120-kD glycoprotein.8
Table 1. Risk Factors
Modifiable Risk Factors
Cholesterol Stone Growth
The nucleated cholesterol monohydrate crystal serves as the nidus for the growth of cholesterol stones.8 Repeated deposition of cholesterol on the nidus leads to stone enlargement. Growth of stones is most likely a discontinuous process that is punctuated by deposition of rings of calcium bilirubinate and calcium carbonate as the daily inter-play of cholesterol, bile salts and phospholipids continues.8 There are several factors that favor cholesterol gallstone formation. The first is cholesterol hypersecretion, which can occur due to advanced age, obesity, hormones (eg, estrogens, progesterone, use of oral contraceptive pills), medications (eg, clofibrate), and marked weight reduction. Pro-nucleating factors (including mucus, glycoproteins, high bile salt to lecithin ratio, high cholesterol to lecithin ratio in vesicles, infections, calcium and bacterial biofilms) and gallbladder stasis (which can occur from total parenteral nutrition, octreotide use, pancretic insufficiency, and spinal cord injury) also increase the risk of cholesterol gallstone formation. Finally, conditions causing a decrease in bile acids, such as ileal disease, bypass or resection of the ileum, primary biliary cirrhosis, congenital 12-hydroxylase deficiency, and cholestyramine can increase the chance of gallstone disease. These factors will be discussed in detail later in this review. Pigment stones make up a small minority and account for approximately 10%30% of gallstone cases in the United States.11,12 This review will not explore in depth the pathogenesis of pigment gallstones due to a lack of epidemiological studies. Briefly, there are 2 types of pigment gall stones: black and brown gallstones. Black pigment stones are predominantly composed of an insoluble bilirubin pigment polymer mixed with calcium phosphate and carbonate.13 Precipitation of calcium bilirubinate occurs whenever the ionic product of calcium and unconjugated bilirubin exceeds its solubility product. The major factors causing black pigment stones are hemolytic anemias, ineffective erythropoiesis, and
Obesity Rapid weight loss Diet Alcohol abstinence Decreased physical activity Smoking Medications (ie, octreotide, ceftriaxone) Hyperlipidemia Diabetes mellitus type II
Unmodifiable Risk Factors
Female Gender Age Genetics
increased production of bilirubin (caused by hereditary spherocytosis, thalassaemia, sickle cell disease, liver cirrhosis, malaria, and ineffective erythropoiesis).13 Factors causing absorption of bilirubin from gut include ileal resection or ileal disease, liver cirrhosis, total parenteral nutrition, and cystic fibrosis.13 Most of the brown pigment stones are formed in the bile ducts as a consequence of some infection and stasis of bile flow.13 Chemically they are calcium salts of long chain fatty acids and cholesterol. Bacterial Bglucuronidase deconjugates bilirubin diglucuronide into insoluble unconjugated bilirubin which precipitates in the bile duct. Any factor that interrupts normal bile flow predisposes to infection, and subsequently to brown pigment stone formation as is observed with biliary strictures, periampullary diverticulum, and Caroli syndrome. Intrahepatic brown pigment stones are seen with infestation of bile ducts with Ascaris lumbricoides and Clonorchis sinensis.13
EPIDEMIOLOGY AND RISK FACTORS Unmodifiable Risk Factors Gender Generally, gallstone disease is more prevalent
in females than in males (Table 1). However, the differences in gallstone incidence between sexes decreases
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
13
The Gallstone Story
DISEASES OF THE BILIARY TRACT, SERIES #4
with advancing age. According to the Group for Epidemiology and Prevention of Cholelithiasis (GREPCO) study, the female-to-male ratio for gallstone disease was 2.9 between 30 to 39 years of age, 1.6 between 40 to 49 years of age, and 1.2 between 50 to 59 years of age.14 Female sex hormones appear to play a role, especially between the ages of 20 and 30 years.5 Another study that researched estrogen receptors and cholesterol biosynthesis found that estrogen in particular stimulated the HMG-Co-A reductase enzyme causing increased synthesis of cholesterol and thus putting women at an increased risk of super saturation. Further supporting the link between estrogen and gallstones, it was determined that postmenopausal women on estrogen replacement therapy were found to have an increased incidence of gallstones.15 Progesterone may also contribute to gallstone disease by inhibiting gallbladder contraction and promoting hypomotility and gallbladder stasis. Parity also appears to be a factor in the development of gallstones. Women with more pregnancies and longer lengths of fertility periods appear to have a higher likelihood of developing gallstones than those who remain nulliparous. A study in Chile found gallstones in 12.2% of multiparous women versus 1.3% of nulliparous women within the same age.16 Another study found women under the age of 25 years with 4 pregnancies were 4 to 12 times more likely to develop cholesterol stones compared to nulliparous women of the same age and weight. It should be noted that biliary sludge usually disappears a few weeks after the pregnancy concludes/terminates/ends.
Table 2. Worldwide Prevalence1,2124
Prevalence (%)
Americas Pima Indians (Arizona) Mupache Indians (Chile) Europe Norway Former East Germany France England Africa Bantu Tribe Asia Northern India China Japan 73 (women >25 years) 90 (women >65 years) 49 (women); 13 (men)
21 19 14 8
6 4 3
Age Age also appears to have an effect on the incidence of gallstone disease. Gallstone disease before 20 years of age is a rare occurrence.5 In infants and children, the most common stones are pigment stones, which are related to hemolysis or chronic diseases such as cystic fibrosis, thalassemia major, and sickle cell anemia.5 Typically, only 0.15% to 0.22% of children will have gallstones, and children account for less than 5% of all cholecystectomies. The increased incidence of gallstones with age is seen across all ethnic groups.5,17 A study in Taiwan confirmed that increasing age had a direct relationship with the development of gallstones simply due to the long-term exposure to other risk fac14
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
tors irrespective of locality or standard of living.17 A Danish study also showed that an increased incidence of gallstone disease in patients 45 years compared with those aged 35 years, while the differences in gallstone incidence between sexes decreased with advancing age.18 From a biochemical standpoint, age itself may increase cholesterol saturation of bile with enhanced hepatic secretion of cholesterol secondary to increased levels of HMG co-A reductase, the rate-limiting enzyme of cholesterol synthesis. Decreased synthesis of bile acids may occur secondary to decreased cholesterol 7 a-hydroxylase enzyme activity, the rate-limiting enzyme in bile acid synthesis, as age advances.19,20
Genetics It remains to be determined the exact role
of genetics in the occurrence of gallstones that would account for the different prevalence rates among the different ethnic groups. However, genetic make-up appears to contribute to the prevalence of cholelithiasis as there is wide variation in incidence between different ethnicities (Table 2).1,2124 For example, the Pima tribe of Arizona has the highest prevalence of
(continued on page 17)
The Gallstone Story
DISEASES OF THE BILIARY TRACT, SERIES #4
(continued from page 14)
gallstone disease in the world, where an estimated 73% of women >25 years have cholelithiasis, with the prevalence rising to 90% by 65 years.21 In South America, the prevalence of gallbladder disease among Mapuche Indians of Chile is 49% in women and 13% in men.22 In the United States, the NHANES III survey showed a higher prevalence of gallstones in MexicanAmericans compared to non-Hispanic whites, while American blacks had the lowest prevalence.1 In Europe, the highest prevalence of gallstone disease was found in Norway (21%) and the former East Germany (19%), while the lowest incidence was found in Italy (6%).23 In Asian countries, the prevalence of cholesterol gallstones is relatively low. These countries and populations are being more commonly associated with pigment stones. In northern India, for example, the prevalence of cholelithiasis is about 6%.24 However, with the increased expansion of the Western diet throughout the world, the number of stones attributed to cholesterol is expected to increase. In individuals, studies have shown that genetic factors are responsible for at least 30% of symptomatic gallstone disease.2527 It has been found that monozygotic twins have been shown to have a higher cholesterol saturation index than dizygotic twins.28 Furthermore, a study in Europe found linked a mutation in the ABCG8-gene with the occurrence of gallstones as this mutation was carried in 21% of 178 men and women with gallstones.29 It was found that this mutated gene was responsible for the pump which transported blood lipid cholesterol from the liver into the bile ducts, therefore, increasing the formation of cholesterol gallstones.
greater with those patients with central obesity and those who developed obesity at an early age rather than in the later years of life.5,31
Rapid Weight Loss Rapid weight loss is another risk
factor for gallbladder disease,5 as demonstrated by the Nurses Health Study,33 which followed close to 90,000 women. This study found that women who lost 4 kg to 10 kg of weight over a 2-year interval had a 44% increase in the risk for gallstone disease as compared with women whose weight changed <4 kg over the same time period. Furthermore, women who lost >10 kg of weight had a 94% increase in the risk for gallstone disease as compared with women whose weight change was <4 kg when controlling for BMI and other risk factors for gallstones. Another study demonstrated that a weight loss >1.5 kg/week led to a dramatic increase in the risk of gallstone formation.34 It has also been shown that sludge and gallstones develop in 25%35% of extremely obese patients with weight loss secondary to bariatric surgery, usually during the first 6 weeks following surgery when weight loss is most profound.35,36
Diet and Activity Closely related to obesity and
rapid weight loss are diet and physical activity. Diets that encompass Vitamin C, moderate alcohol intake, coffee, and nut consumption lower the incidence of gallstone disease (Table 3).5 Conversely, increased cholesterol intake and low fiber diet, typical of Western diets, are predisposing risk factors for the development of gallstone disease.5 This is best exemplified by a study, conducted in Japan after World War II and the
Table 3. Diet5
Modifiable Risk Factors Obesity Obesity is an important risk factor for the
development of gallstone disease. Obese women, defined as a body mass index (BMI) >30 kg/m2 are at twice the risk of gallbladder disease than women with a normal BMI (<25 kg/m2).30 Women with extreme obesity or a BMI >40 kg/m2 have a 7-fold increased risk of gallstones.5,31 The reason for the increased risk of gallstones in obese patients is due to an increased hepatic secretion of cholesterol.5,32 Additionally, the correlation between gallstone disease and obesity is
Increases Risk of Gallstones
High cholesterol/ Low fiber (western diet) Total parenteral nutrition
Lowers Risk of Gallstones
Vitamin C Moderate alcohol intake Coffee Nut consumption 17
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
The Gallstone Story
DISEASES OF THE BILIARY TRACT, SERIES #4
ensuing Westernization of the Japanese diet. That study found that the prevalence of gallstones doubled in Tokyo after the late 1940s with a corresponding increase in pigment-to-cholesterol gallstones.37 Similar increases in gallstone disease have also been seen in other countries, such as Saudi Arabia, where the Western diet has become more widespread.38 Total parenteral nutrition (TPN) also increases the risk of gallstone occurrence as demonstrated by a study that found that the incidence of cholelithiasis was 6.2%, 21.2%, and 38.7% at 6, 12, and 24 months of receiving TPN, respectively.39 With regard to physical activity, decreased activity increases the risk of gallstones, while many forms of activity, including vigorous or brisk walking, protect against gallstone disease.40,41
cans display symptoms and an even greater number remain asymptomatic, thereby making the true prevalence difficult to determine. The underlying pathogenesis appears to be the interaction between cholesterol, phospholipids, and bile salts and the other factors which promote lithogenic bile. It is important to remember that both super saturation of cholesterol in bile and nucleation of cholesterol crystals are needed for the formation of macroscopic gallstones. Risk factors for gallstones which are unmodifiable include female gender through increased estrogen, cholesterol synthesis, age, and genetics. Other risk factors include obesity, rapid weight loss, parity, and diet. Protective factors include vitamin C intake, moderate alcohol intake, coffee, nut consumption, and exercise.
References
1. Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632-639. 2. Sandler RS, Everhart JE, Donowitz M, et al. The burden of seleted digestive dieases in the United States. Gastroenterology. 2002;122:1500-1511. 3. National Institutes of Health Consensus Development of Conference Statement on gallstones and laparoscopic cholecystectomy. Am J Surg. 1993;165:390-398. 4. Portincasa P, Moschetta A,Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230-239. 5. Shaffer EA. Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981-996. 6. Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics. Gastroenterology. 2004;126:1448-1453. 7. Saunders KD, Cates JA, Roslyn JJ. Pathogenesis of gallstones. Surg Clin North Am. 1990;70:1197-1216. 8. Wang HH, Portincasa P, Wang DQ. Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci. 2008;13:401-423. 9. Morrissey KP. Gallstone formation and dissolution. In Clinical Surgery. 1982;16:61-100. 10. Halpern Z, Dudley MA, Kibe A, et al. Rapid vesicle formation and aggregation in abnormal human biles. A time-lapsed videoenhanced contrast microscopy study. Gastroenterology. 1986;90:875-885. 11. Trotman B, Soloway RD. Pigment vs cholesterol cholelithiasis: clinical and epidemiological aspects. Dig Dis. 1975;20:735-740. 12. Trotman BW, Ostrow JD, Soloway RD. Pigment vs cholesterol cholelithiasis: comparison of stone and bile composition. Am J Dig Dis. 1974;19:585-590. 13. Grunhage F, Lammert F. Pathogenesis of gallstones: a genetic perspective. Best Prac Res Clin Gastroenterol. 2006;20:9971015. 14. Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology. 1995;21:655-660. 15. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605-613.
Smoking, Medications, Hperlipidemia, Diabetes Mellitus II There also appears to be a very weak
relationship between gallstones and other common medical conditions such as chronic obstructive pulmonary disease (COPD) and coronary artery disease (CAD). Paradoxically, hyperlipidemia is only tenuously linked to gallstones. Although low levels of high-density lipoprotein (HDL) cholesterol and high triglycerides are associated with gallstone disease,42 the extent to how much the risk is increased has not clearly been defined by any major study. Other diseases such as diabetes mellitus seem to facilitate the development of gallstone formation secondary to increased triglyceride levels associated obesity as well as promoting gallbladder hypomotility and stasis. A study in Japan showed a moderate increase in the risk of gallstones in patients with diabetes mellitus;43 however, there is no conclusive link between these two conditions. Smoking is even less convincingly linked to increased risk of gallstone disease. A multitude of various medications have been linked to the development of gallstones. These include thiazides, oral contraceptives, and ceftriaxone. Somatostatin is also associated with gallstone development because of its effect on impaired gallbladder emptying.
CONCLUSION
In summary, gallstone disease is common among developed countries. Approximately 15% of Ameri18
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
(continued on page 23)
The Gallstone Story
DISEASES OF THE BILIARY TRACT, SERIES #4
(continued from page 18)
16. Valdivieso V, Covarrubias C, Siegel F, Cruz F. Pregnancy and cholelithiasis: pathogensis and natural course of gallstones diagnosed in early puerperium. Hepatology. 1993;17:1-4. 17. Chen CY, Lu CL, Huang YS, et al. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Aging. 1998;27:437-441. 18. Jorgensen T. Prevalence of gallstones in a Danish population. Am J Epidemiol.1987;126:912-921. 19. Einarsson K, Nilsell K, Leijd B, Angelin B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver, N Engl J Med. 1985;313:277-282. 20. Bertolotti M, Bertolotti S, Menozzi D, et al. Ageing and bile acid metabolism: studies on 7a hydroxylation of cholesterol in humans. In: Paumgartner G, Gerok W. eds. Trends in bile acid research. Lancaster: Kluwer Academic Publishers, 1989; 75-78. 21. Sampliner, RE, Bennett, PH, Comess, LJ, et al. Gallbladder disease in pima indians. Demonstration of high prevalence and early onset by cholecystography. N Engl J Med. 1970; 283:1358. 22. Covarrubias C, Valdivieso V, Nervi F. Epidemiology of gallstone disease in Chile. In Capocaccia L, Ricci G & Angelico F, (eds). Epidemiology and prevention of gallstone disease. Lancaster, England: MTP; 1984; 26-30. 23. Aerts R, Penninckz F. The burden of gallstone disease in Europe. Aliment Paramacol Ther. 2003;18:49-53. 24. Khuroo MS, Mahajan R, Zargar SA, Javid G, Sapru S. Prevalence of biliary tract disease in India: a sonographic study in adult population in Kashmir. Gut. 1989;30:201-205. 25. Miquel JF, Covarrubias C, Villaroel L, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115:937-946. 26. Carey MC, Paigen B. Epidemiology of the American Indians burden and its likely genetic origins. Hepatology. 2002;36:781791. 27. Lammert F, Sauerbruch T. Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat Clin Prac Gastroenterol Hepatol. 2005;2:423-433. 28. Kesaniemi YA, Koskenvuo M, Vuoristo M, et al. Biliary lipid composition in monozygotic and dizygotic pairs of twins. Gut. 1989;30:1750-1756. 29. Grunhage F, Acalovschi M, Tirziu S. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793801. 30. Everhart JE. Contributions of obesity and weight loss to gallstone disease. Ann Intern Med. 1993;119:1029-1035. 31. Sahi T, Puffenbarger RS, Hseih C, et al. Body mass index, cigarette smoking and other characteristics as predictors of selfreported, physician-diagnosed gallbladder disease in male college alumni. Am J Epidemiol. 1998;147:644-651. 32. Shaffer EA, Small DM. Biliary lipid secretion in chilesterolgallstone disease. The effect of cholecystectomy and obesity. J Clin Invest. 1977;59:828-840. 33. Stampfer MJ, Maclure KM, Colditz GA, Manson JE, Willett WC. Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr. 1992;55:652-658. 34. Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med. 1995;98:115-117. 35. Shiffman ML, Sugerman HJ, Kellum JM et al. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000-1005. 36. Al-Jiffry BO, Shaffer EA, Saccone GTP, et al. Chages in gallbladder motility and gallstone formation following laparoscopic gastric banding for morbid obesity. Can J Gastroenterol. 2003;17:169-174. 37. Kameda H, Ishihara F, Shibata K, et al. Clinical and nutritional study on gallstone disease in Japan. Jpn Med J. 1984;23:109-113. 38. Murshid KR. Symptomatic gallstones: A disease of young Saudi women. Saudi J Gastroenterol. 1998;4:159-162. 39. Dray X, Joly F, Reijasse D, et al. Incidence, risk factors, and complications of cholelithiasis in patients with home parenteral nutrition. J Am Coll Surg. 2007;204:13-21. 40. Leitzmann MF, RImm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777-784. 41. Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417-425. 42. The Rome Group for Epidemiology and Prevention of Cholelithiasis (GREPCO). The epidemiology of gallstone disease in Rome, Italy. Part II. Factors associated with the disease. Hepatology. 1988;8:907-913. 43. Shizuka S, Kono S, Todoroki I, et al. Impaired glucose tolerance, diabetes mellitus and gallstone disease: an extended study of male self-defense officials in Japan. Eur J Epidemiol. 1999;15:245-251.
Fellows Corner
is open to
Trainees and Residents ONLY.
Section Editor: C. S. Pichumoni, M.D.
Send in a brief case report. No more than one double-spaced page. One or two illustrations, up to four questions and answers and a three-quarter to one-page discussion of the case. Case to include no more than two authors. A $100.00 honorarium will be paid per publication.
Case should be sent to:
C. S. Pitchumoni, M.D. Chief, Gastroenterology, Hepatology and Clinical Nutrition St. Peters University Hopsital 254 Easton Avenue, Box 591 New Brunswick, NJ 08903 E-mail: pitchumoni@hotmail.com
PRACTICAL GASTROENTEROLOGY SEPTEMBER 2010
23
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Abdominal PainDocument26 pagesAbdominal Painsammy_d6No ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Complete Abdomen Pelvic UltrasoundDocument3 pagesComplete Abdomen Pelvic Ultrasoundሀይደር ዶ.ርNo ratings yet
- Multiple Choice Questions of Radiology and ImagingDocument18 pagesMultiple Choice Questions of Radiology and ImagingVishakha Wahule90% (10)
- Gallbladder Health Julie Daniluk's 13 Paths To Save Your Gallbladder NaturallyDocument7 pagesGallbladder Health Julie Daniluk's 13 Paths To Save Your Gallbladder NaturallyNus EuNo ratings yet
- Nur 102 Lecture Sas 13-23Document14 pagesNur 102 Lecture Sas 13-23Bea SaraumNo ratings yet
- Internal DiseaseDocument55 pagesInternal Diseaseshilpa sekhar278No ratings yet
- Pancreatic PathologyDocument7 pagesPancreatic Pathologyzeroun24100% (1)
- Obstructive Jaundice Secondary To CholedocholithiasisDocument94 pagesObstructive Jaundice Secondary To CholedocholithiasisMacky75% (4)
- CHOLECYSTOLITHIASISDocument22 pagesCHOLECYSTOLITHIASISMc N Mi KabilingNo ratings yet
- Ignatavicius Chapter 59 (Evolve), Ignatavicius Chapter 57 (Evolve), GI CH 56, 57, 58, 59 Ignatavicius, Ignatavicius Chapter 54 (Evolve), Sole Ch 19, Ignatavicius Chapter 63 (Evolve), Sole Ch 16 Flashcards _ QuizletDocument63 pagesIgnatavicius Chapter 59 (Evolve), Ignatavicius Chapter 57 (Evolve), GI CH 56, 57, 58, 59 Ignatavicius, Ignatavicius Chapter 54 (Evolve), Sole Ch 19, Ignatavicius Chapter 63 (Evolve), Sole Ch 16 Flashcards _ QuizletNursyNurseNo ratings yet
- Comorbidities and Complications of Obesity in Children and Adolescents - UpToDateDocument35 pagesComorbidities and Complications of Obesity in Children and Adolescents - UpToDatemarina alvesNo ratings yet
- Askep Klien DG CholelithiasisDocument13 pagesAskep Klien DG CholelithiasisAmanda Mahendra SastranegaraNo ratings yet
- Know About Gall Bladder IntroductionDocument4 pagesKnow About Gall Bladder Introductionfredrickstorrs01No ratings yet
- Case Study CholecystitisDocument13 pagesCase Study Cholecystitissanthyakunjumon100% (1)
- Dotaverine HCLDocument5 pagesDotaverine HCLalbertsmasudoNo ratings yet
- Open CholecystectomyDocument10 pagesOpen CholecystectomyMaria Teresa VillanuevaNo ratings yet
- (Signed) 2021 Team Ventura Contract-R5-08172019Document11 pages(Signed) 2021 Team Ventura Contract-R5-08172019Mahmoud Ahmed TawfikNo ratings yet
- Test - Surgery Book MCQs - Final Exam - QuizletDocument158 pagesTest - Surgery Book MCQs - Final Exam - Quizlety SNo ratings yet
- Síndrome de MirizziDocument5 pagesSíndrome de MirizziOmar DorantesNo ratings yet
- Medical Terminology A Living Language 5Th Edition Fremgen Test Bank Full Chapter PDFDocument67 pagesMedical Terminology A Living Language 5Th Edition Fremgen Test Bank Full Chapter PDFdonaldbau62h100% (11)
- Gallbladder 101Document69 pagesGallbladder 101Gerry OshNo ratings yet
- Biliary Tract: Dr. R. Bhuvanamha Devi Associate Professor Dept of Pathology SRM MCH & RC, PotheriDocument18 pagesBiliary Tract: Dr. R. Bhuvanamha Devi Associate Professor Dept of Pathology SRM MCH & RC, PotheriBhuvanamha Devi ramamurthyNo ratings yet
- PGD CNAD 607 Meal Management and Planning Disease SheetDocument14 pagesPGD CNAD 607 Meal Management and Planning Disease SheetHasaan QaziNo ratings yet
- Best Practice Guidelines and Cochrane Reviews in Gastroenterology and Hepatology-2019Document573 pagesBest Practice Guidelines and Cochrane Reviews in Gastroenterology and Hepatology-2019Engin UçarNo ratings yet
- Acute Pancreatitis SymptomsDocument12 pagesAcute Pancreatitis SymptomsUthraa SalvatoreNo ratings yet
- GIT - Dr. Allam 2021 PDFDocument47 pagesGIT - Dr. Allam 2021 PDFMohammedNo ratings yet
- Gastroenterology - GallbladderDocument2 pagesGastroenterology - GallbladderEugen MNo ratings yet
- Deadman Mazin Al Khafaji Kevin BakerDocument34 pagesDeadman Mazin Al Khafaji Kevin Bakermelho.cottonNo ratings yet
- CholecystectomyDocument6 pagesCholecystectomyTom Bayubs-tucsNo ratings yet
- Acute PancreatitisDocument9 pagesAcute PancreatitisestefygomezsNo ratings yet