Professional Documents
Culture Documents
Chemical Kinetics: Describing The Rate of A Reaction
Uploaded by
Irfan ShafiqOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical Kinetics: Describing The Rate of A Reaction
Uploaded by
Irfan ShafiqCopyright:
Available Formats
Chemical Kinetics
Part I
Describing the Rate of a Reaction
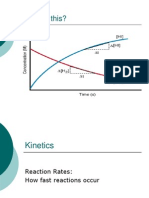
What is Kinetics?
What Is It Good For?
Kinetics is the study of reaction rates.
Information about reaction rates
allows us to predict how long it will take for a reaction
to reach completion
tells us how the reaction proceeds at the molecular level
(mechanism)
Understanding reaction mechanisms provides chemists with
important insight into how reactions work, and also provides a
basis for designing new reactions and processes.
Measuring Reaction Rates
Image from http://www.cstl.nist.gov/div838/crds_web/gas_app.htm
An apparatus designed to measurement very fast reaction rates in gases
(NIST Experimental Chemical Kinetics Thermodynamics Division)
Factors Affecting Rate of a Reaction
Reaction conditions
Concentration (solutions)
Surface area (solids)
Temperature
Chemical effects
Nature of reactants (reaction mechanism)
Catalysts
Describing the Rate of a Reaction
Reaction rate and rate law
Order of a reaction
Dependence on concentration and Time
Half life
Defining Rate
2NO
2
(g) 2NO(g) + O
2
(g)
Rate is expressed as a positive number!
Rate of appearance of product NO
Rate of disappearance of reactant NO
2
unit time per ion concentrat in change = =
dt
dC
Rate
| |
dt
NO d
Rate=
dt
NO d
Rate
] [
2
=
Measuring Rate
Instantaneous Rate
For disappearance of NO
2
Rate = -(slope of tangent)
2NO
2
(g) 2NO(g) + O
2
(g)
Average rate
( )
1 1 5
10 1 . 2
) 0 300 (
/ 0100 . 0 0038 . 0
=
=
=
s L mol
s
L mol
Rate
| |
1 1 5
2
10 4 . 2
110
) / 0026 . 0 (
=
=
A
A
=
s L mol
s
L mol
t
NO
Rate
At t = 100 s
For average rate from 0 and 300 s, use
actual values of [NO
2
] at these times.
100 s
0.0038
Relating Rates for Reactants and Products
Relative rate depends on
stoichiometry
Reaction rate must be reported
with respect to a specific
reactant or product!
2NO
2
(g) 2NO(g) + O
2
(g)
| | | | | |
t
O
t
NO
t
NO
A
A
=
A
A
=
A
A
2 2
2
For NO
2
reaction
1 1 6
1 1 6
10 3 . 4
10 6 . 8
2
2
=
= =
s L mol R
s L mol R R
O
NO NO
At 250 s, the rates are
250 s
Rate and Concentration
Rate Law and Reaction Order
Method of Initial Rates
Rate Law and Reaction Order
For the reaction
aA + bB products
The rate law is
| | | |
n m
B A k Rate =
k = rate constant
m = order w.r.t. A
n = order w.r.t. B
Overall order = m + n
Determine by
experiment
Determining the Rate Law
Goal
Determine rate constant k and orders m and n.
Two approaches
Method of initial rates
Plots of concentration vs time
NO
2
(g) + CO(g) NO(g) + CO
2
(g)
| | | |
n m
CO NO k Rate
2
=
Reaction Order by Method of Initial
Rates
Goal: Determine rate constant k and orders m and n.
To determine m, observe effect of [NO
2
] on rate while holding
[CO] constant.
To determine n, observe effect of [CO] on rate while holding
[NO
2
] constant.
Once m and n are known, calculate k directly from rate equation
and experimental data.
NO
2
(g) + CO(g) NO(g) + CO
2
(g)
| | | |
n m
CO NO k Rate
2
=
Method of Initial Rates - Example
NO
2
(g) + CO(g) NO(g) + CO
2
(g)
| | | |
n m
CO NO k Rate
2
=
Trial [CO] [NO
2
] Rate
mol/L s
1 0.10 0.10 0.005
2 0.10 0.20 0.010
3 0.20 0.10 0.010
| | | |
| | | |
1
2
1
2
1
20 . 0
10 . 0
010 . 0
005 . 0
2 and 1 For trials
2 2 2
1 1 2
2
1
=
|
.
|
\
|
=
|
.
|
\
|
=
=
m
CO NO k
CO NO k
R
R
m
m
n m
n m
To determine m
observe effect of [NO
2
] on
rate while holding [CO]
constant.
Method of Initial Rates - Example
NO
2
(g) + CO(g) NO(g) + CO
2
(g)
| | | |
n m
CO NO k Rate
2
=
Trial [CO] [NO
2
] Rate
mol/L s
1 0.10 0.10 0.005
2 0.10 0.20 0.010
3 0.20 0.10 0.010
| | | |
| | | |
1
2
1
2
1
20 . 0
10 . 0
010 . 0
005 . 0
2 and 1 For trials
2 2 2
1 1 2
2
1
=
|
.
|
\
|
=
|
.
|
\
|
=
=
n
CO NO k
CO NO k
R
R
n
n
n m
n m
To determine n
observe effect of [CO]
on rate while holding
[NO
2
] constant.
( ) ( )
( )
( )
( )
1
5 . 0 log
5 . 0 log
5 . 0 log
5 . 0 log 5 . 0 log
= =
=
=
n
n
n
For more difficult
cases, take logarithm
of both sides
Method of Initial Rates - Example
NO
2
(g) + CO(g) NO(g) + CO
2
(g)
| | | |
n m
CO NO k Rate
2
=
Trial [CO] [NO
2
] Rate
mol/L s
1 0.10 0.10 0.005
2 0.10 0.20 0.010
3 0.20 0.10 0.010
We have determined that m = 1
and n = 1. Using data from trial 1,
we can calculate k.
| | | |
( ) ( )
s mol L
L mol L mol
s L mol
CO NO
Rate
k
n m
/ 50 . 0
/ 10 . 0 / 10 . 0
/ 005 . 0
1 1
2
=
=
=
Summary results
the reaction is 1
st
order wrt NO
2
the reaction is 1
st
order wrt CO
the rate constant k = 0.50 L/mol s
the rate law is
| || | CO NO k Rate
2
=
Relations Between Concentration
and Time
Integrated form of the rate law
Graphical method for determination of the
order of a reaction
Half-life and rate constant
Derivation of the Integrated Rate Law
A Products
Two equations for rate:
| |
dt
A d
Rate =
Equate the right-hand sides:
(Differential form of rate law)
| |
| |
n
A k
dt
A d
=
Integrate from [A]
0
to [A] and t = 0 to t,
where A
0
is initial [A] at t =0:
(I ntegral form of rate law)
Rearranging,
| |
| |
} }
=
t A
A
n
dt k
A
A d
0
0
| |
| |
kdt
A
A d
n
=
| |
n
A k Rate =
Relations Between Concentration and Time
Expressions for the Integrated Rate Law
| |
| |
} }
=
t A
A
n
dt k
A
A d
0
0
Zero order
Rate = k
(n = 0)
| | | |
| | | | kt A A
or
kt A A
=
=
0
0
[A]
t
-k
First order
Rate = k[A]
(n = 1)
| |
| |
| | | | kt A A
or
kt
A
A
=
=
0
0
ln ln
ln
ln[A]
t
-k
Second order
Rate = k[A]
2
(n = 2)
| | | | ( )
| | | |
kt
A A
or
kt A A
+ =
=
0
1
0
1
1 1
1/[A]
t
k
A products
Relations between concentration and time
Expressions for the Integrated Rate Law
Zero order:
First order:
Second order:
To determine reaction order w.r.t. A, generate plots.
If [A] vs t is linear, reaction is 0
st
order.
If ln[A] vs t is linear, reaction is 1
st
order.
If 1/[A] vs t is linear, reaction is 2
nd
order.
| | | | kt A A =
0
| | | | kt A A =
0
ln ln
| | | |
kt
A A
+ =
0
1 1
Graphical Determination of Reaction Order
A products
If zero order, plot of [A] vs t should be linear.
Zero Order Plot
0.000
0.020
0.040
0.060
0.080
0.100
0.120
0 10 20 30 40 50
time, s
[
A
]
time, s [A] ln[A] 1/[A]
0 0.100 -2.303 10.0
10 0.067 -2.703 14.9
20 0.045 -3.103 22.3
30 0.030 -3.503 33.2
40 0.020 -3.903 49.5
| | | | kt A A =
0
Not zero order
Graphical Determination of Reaction Order
A products
If first order, plot of ln[A] vs t should be linear.
First Order Plot
-4.000
-3.800
-3.600
-3.400
-3.200
-3.000
-2.800
-2.600
-2.400
-2.200
-2.000
0 10 20 30 40 50
time, s
l
n
[
A
]
time, s [A] ln[A] 1/[A]
0 0.100 -2.303 10.0
10 0.067 -2.703 14.9
20 0.045 -3.103 22.3
30 0.030 -3.503 33.2
40 0.020 -3.903 49.5
| | | | kt A A =
0
ln ln
Reaction is first order!
Graphical Determination of Reaction Order
A products
If second order, plot of 1/[A] vs t should be linear.
Second Order Plot
0.0
10.0
20.0
30.0
40.0
50.0
60.0
0 10 20 30 40 50
time, s
1
/
[
A
]
time, s [A] ln[A] 1/[A]
0 0.100 -2.303 10.0
10 0.067 -2.703 14.9
20 0.045 -3.103 22.3
30 0.030 -3.503 33.2
40 0.020 -3.903 49.5
| | | |
kt
A A
+ =
0
1 1
Not second order!
Graphical Determination of Rate Law
2H
2
O
2
2H
2
O + O
2
Data
Time (s) [H2O2] (mol/L)
0 1.00
120 0.91
300 0.78
600 0.59
1200 0.37
1800 0.22
2400 0.13
3000 0.082
3600 0.050
Generate the following plots in Excel
[H
2
O
2
] vs t
ln[H
2
O
2
] vs t
1/[H
2
O
2
] vs t
see Excel spreadsheet
The plot of ln[H
2
O
2
] vs t yields a
straight line. Reaction is first order!
What is
the rate law for the reaction
the integrated rate law
the value of the rate constant
[H
2
O
2
] at 4000 s
Half-Life
Half-life is the time required for the concentration
of a reactant to decrease by a factor of 2.
t
1/2
= half-life
Many reactions, including radioactive processes,
are first order. Lets examine the quantitative
aspects of half-life for a first-order reaction.
A products
Rate = k[A]
Half-Life for a 1st Order Reaction
A products
t
1/2
= time for [A] to decrease by factor of 2.
0.000
0.020
0.040
0.060
0.080
0.100
0.120
0 10 20 30 40 50
time, s
[
A
]
t
1/2
=10 s
time, s [A] n 2^n
0 0.100 0 1.0
10 0.050 1 2.0
20 0.025 2 4.0
30 0.013 3 8.0
40 0.006 4 16.0
Data for a reaction with a 10-s half-life
After n half-lives,
[A] = [A]
0
/2
n
Relating t
1/2
and k
For the 1st order reaction A products
Rate = k[A]
Noting that [A] = [A]
0
/2 when t = t
1/2
| | | |
| |
| | A
A
A A kt
kt A A
0
0
0
ln ] ln[ ] ln[
ln ln
= =
=
k k
t
kt
693 . 0 2 ln
2 ln
2 / 1
2 / 1
= =
=
Calculations Involving Half-Life for a
First-Order Reaction
The radioactive isotope
32
P decays by first-order kinetics, t
1/2
= 14.3 days. How
long does it take for 95.0% of a given sample of
32
P to decay?
32
P products
Rate = k[
32
P]
| | | | kt A A =
0
ln ln
k k
t
693 . 0 2 ln
2 / 1
= =
For a 1
st
order-reaction, expressions for the integrated rate law and t
1/2
are
1
2 / 1
0485 . 0 3 . 14 693 . 0 2 ln
= = = d d t k
The rate constant is calculated from t
1/2
| | | | ( ) | | | | ( )
days
d k
A A
k
A A
t 8 . 61
0484 . 0
20 ln 050 . 0 ln ln
1
0 0 0
= = = =
At 95.0% decay, [A] = 0.050[A]
0
. Solving the integrated rate law for t
Summary of Kinetics I
Describing the Rate of Reaction
Kinetics tells us
how fast reaction
proceeds
mechanism of reaction
Factors affecting rate
Describing rate
Rate = -dC/t
aA + bB products
Summary of Kinetics I
Describing the Rate of Reaction
Kinetics tells us
how fast reaction proceeds
mechanism of reaction
Factors affecting rate
Reaction conditions
C, T, surface area
Nature of reactants
Catalysts
Describing rate
Rate = -A[A]/At
Instantaneous rate, average rate
Rate and Concentration
Rate Law
Rate = k[A]
m
[B]
n
Method of initial rates
to determine k, m, n
Concentration and time
Integrated rate laws
zero order
[A] = [A]
0
- kt
first order
ln[A] = ln[A]0 - kt
second order
1/[A] = 1/[A]
0
+ kt
Graphical determination of
order and rate constant
Half-life and rate constant
t
1/2
= ln2/k
aA + bB products
You might also like
- S650 Service - 6987168 enUS SMDocument1,311 pagesS650 Service - 6987168 enUS SMcarlos andres salazar sanchez75% (4)
- Ab Initio Interview Questions - HTML PDFDocument131 pagesAb Initio Interview Questions - HTML PDFdigvijay singhNo ratings yet
- Safety in Manufacturing: Ergonomics: Awkward PosturesDocument2 pagesSafety in Manufacturing: Ergonomics: Awkward PosturesprashanthNo ratings yet
- Construction of Dormitory & Housing compounds in NorochcholaiDocument33 pagesConstruction of Dormitory & Housing compounds in Norochcholaisaranga100% (1)
- 11 Reaction KineticsDocument95 pages11 Reaction KineticsSyamil Adzman100% (1)
- Make $50 A Day Autopilot MethodDocument4 pagesMake $50 A Day Autopilot MethodJadon BoytonNo ratings yet
- Chap 8 Reaction Kinetics 1415FARRADocument129 pagesChap 8 Reaction Kinetics 1415FARRA黄麒安No ratings yet
- Delem: Installation Manual V3Document73 pagesDelem: Installation Manual V3Marcus ChuaNo ratings yet
- Motion For Bill of ParticularsDocument3 pagesMotion For Bill of ParticularsPaulo Villarin67% (3)
- Approvals Management Responsibilities and Setups in AME.BDocument20 pagesApprovals Management Responsibilities and Setups in AME.BAli LoganNo ratings yet
- Chapter 2 - Chemical KineticsDocument92 pagesChapter 2 - Chemical KineticsJohan Daniyal100% (1)
- 102 MSJC 13Document11 pages102 MSJC 13noelNo ratings yet
- 6 - Chemical Kinetics PDFDocument16 pages6 - Chemical Kinetics PDFthinkiit100% (1)
- Reaction KineticsDocument37 pagesReaction KineticsNurshuhada NordinNo ratings yet
- Chem. KineticsDocument51 pagesChem. KineticsShyam Singh SainiNo ratings yet
- Rate LawsDocument19 pagesRate LawsEli BerkowitzNo ratings yet
- 15 Chem KinetDocument51 pages15 Chem KinetRoua Ali100% (2)
- Chemical KineticsDocument41 pagesChemical Kineticskishangopi1230% (1)
- Apchapt 12Document87 pagesApchapt 12Syed NazrinNo ratings yet
- Unit 15 Kinetics: ChemicalDocument75 pagesUnit 15 Kinetics: Chemicalpetercyh175No ratings yet
- For Chapter 5555Document58 pagesFor Chapter 5555bahru demekeNo ratings yet
- Chemical Kinetics Part IDocument45 pagesChemical Kinetics Part IKarthikanAmirthalingamNo ratings yet
- Chemical Kinetic Note 03Document28 pagesChemical Kinetic Note 03Nurul Izzanie AdnanNo ratings yet
- Overview of KineticsDocument13 pagesOverview of Kineticsbits_who_am_iNo ratings yet
- Chemical Kinetics1Document59 pagesChemical Kinetics1farooq_bagbanNo ratings yet
- Physical Chemistry - KineticsDocument66 pagesPhysical Chemistry - KineticsarieleliannasternNo ratings yet
- Chem Chapt13 PractiseDocument5 pagesChem Chapt13 PractiseqwerNo ratings yet
- Kinetika KimiaDocument39 pagesKinetika KimiaMia YukimuraNo ratings yet
- Chemical KineticsDocument7 pagesChemical Kineticsthinkiit100% (1)
- ObjectivesDocument24 pagesObjectivesAvicenna Ibnu BahrinNo ratings yet
- Chemical Kinetics: General Chemistry 2Document27 pagesChemical Kinetics: General Chemistry 2France TurdaNo ratings yet
- Advancedchemistry-Lecture Slides-Kinetics Lessons Student VersionDocument26 pagesAdvancedchemistry-Lecture Slides-Kinetics Lessons Student VersionJavier Blanco AlvarezNo ratings yet
- Chem Chapter 13 LECDocument103 pagesChem Chapter 13 LECsaxman011No ratings yet
- Chem Kinet Meeting 2Document27 pagesChem Kinet Meeting 2Nuril AzmiNo ratings yet
- Kinetics ReactionDocument40 pagesKinetics ReactionSohila A. MabroukNo ratings yet
- Chemical Kinetics: Rate of Reaction MechanismDocument60 pagesChemical Kinetics: Rate of Reaction MechanismAneudis Javier BritoNo ratings yet
- SM Chapter 15Document51 pagesSM Chapter 15李承家No ratings yet
- Reaction KineticsDocument37 pagesReaction KineticsDaisyNo ratings yet
- ChE441 Analysis of Rate Data-1Document28 pagesChE441 Analysis of Rate Data-1Xnd3RNo ratings yet
- Lecture 14 Slides Kinetics Week 5-2 PDFDocument34 pagesLecture 14 Slides Kinetics Week 5-2 PDFSam YuritrevNo ratings yet
- Chp13, Rate Law DeterminDocument9 pagesChp13, Rate Law DeterminrofikudouNo ratings yet
- Rate LawsDocument20 pagesRate LawsReginal MoralesNo ratings yet
- Matriculation Chemistry (Reaction Kinetics) Part 2Document18 pagesMatriculation Chemistry (Reaction Kinetics) Part 2ridwan100% (2)
- 16 Reaction Kinetics II PDFDocument9 pages16 Reaction Kinetics II PDFjenatNo ratings yet
- Chemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaDocument34 pagesChemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, Canadadescar84No ratings yet
- Chem KineticsDocument32 pagesChem KineticsmojakovichNo ratings yet
- CHM 112 Kinetics Practice Problems Answers - Reader ViewDocument19 pagesCHM 112 Kinetics Practice Problems Answers - Reader ViewSyasya FaqihahNo ratings yet
- Main Factors Which Influence Reaction Rate:: Concentrations of Reactants Reaction Temperature Presence of A CatalystDocument32 pagesMain Factors Which Influence Reaction Rate:: Concentrations of Reactants Reaction Temperature Presence of A CatalystsareddyNo ratings yet
- Lecture 3 Kinetics Part 2Document82 pagesLecture 3 Kinetics Part 2Yahmeela SernaNo ratings yet
- RXN RatesDocument22 pagesRXN RatesDana CapbunNo ratings yet
- CH4002 L12-16 2017 KH Chemical Kinetics IntroductionDocument56 pagesCH4002 L12-16 2017 KH Chemical Kinetics Introductionjesuslovers3000No ratings yet
- CHM 207 Kinetics Lecture 3Document8 pagesCHM 207 Kinetics Lecture 3Oluwatosin OsisanyaNo ratings yet
- Rates of Reaction Factors and EquationsDocument10 pagesRates of Reaction Factors and Equationsginell grantNo ratings yet
- Week 2. Chemical Kinetics Analysis of Rate EquationDocument31 pagesWeek 2. Chemical Kinetics Analysis of Rate EquationYuni ApriyaniNo ratings yet
- Chapter 14 Lecture NotesDocument59 pagesChapter 14 Lecture NotesDavis LundNo ratings yet
- Determining The Rate Law From Experimental DataDocument45 pagesDetermining The Rate Law From Experimental Datasospeter barasaNo ratings yet
- CH 7 - Chemical KineticsDocument60 pagesCH 7 - Chemical KineticsCharbel RahmeNo ratings yet
- Chapter 2 Chemical KineticsDocument83 pagesChapter 2 Chemical KineticsRaymond KakalaNo ratings yet
- Chapter 6Document46 pagesChapter 6Yahya Alhaddi KA201 18No ratings yet
- Topic 7Document6 pagesTopic 7Bert ManNo ratings yet
- SCH 305Document34 pagesSCH 305Qwin NajyaNo ratings yet
- Chemical KineticsDocument51 pagesChemical KineticsdkaurNo ratings yet
- Kinetic Concepts of Heterogeneous Photocatalysis: Background SketchDocument30 pagesKinetic Concepts of Heterogeneous Photocatalysis: Background SketchEvangelina Valencia HernándezNo ratings yet
- Chemical Kinetics: Practice ExamplesDocument31 pagesChemical Kinetics: Practice ExamplesJudith Del Valle MorejonNo ratings yet
- Chemical KineticsDocument62 pagesChemical KineticsIbnu Abbas Al BasthomiNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Fundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsFrom EverandFundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Iso 18254 - 2016-ApeoDocument18 pagesIso 18254 - 2016-ApeoIrfan ShafiqNo ratings yet
- CPSC-CH-C1001-09.4 Standard Operating Procedure For Determination of PhthalatesDocument8 pagesCPSC-CH-C1001-09.4 Standard Operating Procedure For Determination of PhthalatesIrfan ShafiqNo ratings yet
- Agilent 5973 GCMS Training Manual: SafetyDocument11 pagesAgilent 5973 GCMS Training Manual: SafetyIrfan ShafiqNo ratings yet
- Chemical Kinetics: Describing The Rate of A ReactionDocument29 pagesChemical Kinetics: Describing The Rate of A ReactionIrfan ShafiqNo ratings yet
- Foundations of Engineering Economy: Graw HillDocument132 pagesFoundations of Engineering Economy: Graw HillMohd KarimNo ratings yet
- Evaporator Types GuideDocument22 pagesEvaporator Types GuideIrfan ShafiqNo ratings yet
- Irfan ShafiqDocument21 pagesIrfan ShafiqIrfan ShafiqNo ratings yet
- Ceoeg-Cebqn Rev0Document3 pagesCeoeg-Cebqn Rev0jbarbosaNo ratings yet
- Mecafix 120: Description Technical DataDocument1 pageMecafix 120: Description Technical DataJuan Carlos EspinozaNo ratings yet
- Lessee Information StatementDocument1 pageLessee Information Statementmja.carilloNo ratings yet
- Black Box Components and FunctionsDocument9 pagesBlack Box Components and FunctionsSaifNo ratings yet
- A Study On Capital BudgetingDocument2 pagesA Study On Capital BudgetingANKIT SINGHNo ratings yet
- DPD 2Document1 pageDPD 2api-338470076No ratings yet
- CASE FLOW AT REGIONAL ARBITRATIONDocument2 pagesCASE FLOW AT REGIONAL ARBITRATIONMichael Francis AyapanaNo ratings yet
- Keys and Couplings: Definitions and Useful InformationDocument10 pagesKeys and Couplings: Definitions and Useful InformationRobert Michael CorpusNo ratings yet
- Ubaf 1Document6 pagesUbaf 1ivecita27No ratings yet
- Manual Mue Home RGBDocument8 pagesManual Mue Home RGBJason OrtizNo ratings yet
- FINC 301 MQsDocument40 pagesFINC 301 MQsMichael KutiNo ratings yet
- Panameterics GF 868 Flare Gas Meter PDFDocument8 pagesPanameterics GF 868 Flare Gas Meter PDFDaniel DamboNo ratings yet
- Nº SSR-1 NS-R-3 Draf R1 Site Evaluation For Nuclear Installations FRDocument33 pagesNº SSR-1 NS-R-3 Draf R1 Site Evaluation For Nuclear Installations FRdaniel addeNo ratings yet
- Mom Luby and The Social WorkerDocument1 pageMom Luby and The Social WorkerqtissskrazyNo ratings yet
- Manual Circulação Forçada PT2008Document52 pagesManual Circulação Forçada PT2008Nuno BaltazarNo ratings yet
- Chapter FiveDocument12 pagesChapter FiveBetel WondifrawNo ratings yet
- QA InspectionDocument4 pagesQA Inspectionapi-77180770No ratings yet
- Presenting India's Biggest NYE 2023 Destination PartyDocument14 pagesPresenting India's Biggest NYE 2023 Destination PartyJadhav RamakanthNo ratings yet
- QAQC Inspection Services Technical Proposal SummaryDocument69 pagesQAQC Inspection Services Technical Proposal SummaryMathias OnosemuodeNo ratings yet
- Infineon ICE3BXX65J DS v02 - 09 en PDFDocument28 pagesInfineon ICE3BXX65J DS v02 - 09 en PDFcadizmabNo ratings yet
- 2011 REV SAE Suspension Kiszco PDFDocument112 pages2011 REV SAE Suspension Kiszco PDFRushik KudaleNo ratings yet
- Examples 5 PDFDocument2 pagesExamples 5 PDFskaderbe1No ratings yet