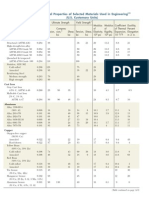
Professional Documents
Culture Documents
Aas 2 PDF
Uploaded by
Sachith Praminda RupasingheOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aas 2 PDF
Uploaded by
Sachith Praminda RupasingheCopyright:
Available Formats
Atomic Absorption Spectrophotometry (AAS)
When using atomic absorption spectrophotometry (AAS) as an analytical technique the absorption of light of free atoms is measured. Therefore it is one of the branches of atomic spectroscopy, together with flame photometry (see Standardbase techniques: Flame Photometry that measures the intensity of light emitted by free atoms when their electrons return to ground state after the excitation by light). However - unlike flame photometry - AAS is based on the first half of the excitation process, while atoms absorb light getting their electrons from the ground state to a higher energy level. Although the atomic absorption spectrophotometer (fig. 1.) is quite expensive, the technique is very wide-spread, thank to the fact that by AAS it is possible to determine about 70 elements (mainly metals) at very low concentrations. The sample is atomised at a very high temperature (2500-3000 C) and the free atoms have line spectrum. It means that they can only absorb the energy of light at discrete energy levels according to the excitations of electrons. Excitation energies in this case are determined by the difference between the energy level of the ground state and one of the excitation states of their electrons. Only a light with a concrete wavelength belongs to each of these excitation energies and when this light is absorbed it is missing from the continuous spectra of the electromagnetic radiation: a black line appears in the absorption spectrum of the atom. There are no vibration or rotation energy levels that would widen the lines to brands in the spectrum (like it happens in the case of UV-Vis spectrophotometry, when molecules and ions are measured, see Standardbase technique: UV-Vis Spectrophotometry). Using AAS free atoms are lit by monochromatic light (called resonance radiation that has got a special wavelength) that belongs to one line of their spectrum and therefore it has the suitable excitation energy mentioned above. Only the examined atoms can absorb it. As a result of absorption, the intensity of light decreases, which is proportional to the number of the examined atoms being present. That makes very sensitive quantitative measurements possible.
Figure 1: Photograph of an atomic absorption spectrophotometer
To produce the proper monochromatic light necessary for the AAS, so called hollow cathode lamps are used. The cathode of this sort of lamp is made of the metal under investigation (or its alloy). It means that different lamps are used for the determination of each element. It is named after the cylindrical shape of the cathode that gives direction to emerging beam, and helps re-deposit sputtered atoms back on cathode. The anode is made of tungsten and the electrodes are surrounded by noble gases. At high voltage the cathode produces electrons that speeding up in the electric filed cause the ionisation of noble gas atoms. These high-speed noble gas ions bombard the cathode and therefore sputtering occurs, dislodging atoms from the surface of cathode. These free atoms are excited by the high-speed electrons and then emit the line spectrum characteristic of the particular element that is the cathode made of. AAS called a destructive technique, because only solutions containing the investigated element can be used. Solid samples should be accurately weighed and then dissolved, often using strong acids (e.g. in cases when soil samples contaminated with heavy metal ions are measured). However only a very small amount of sample is enough, because of the high sensitivity of the technique. The solvent of the solution is evaporated and all materials present in the sample are vaporised and dissociated to atoms at the very high temperature. (The process in the reality is a bit more complicated, since ions and oxides are also produced, decomposition and association reactions take place too.) The following atomisation methods are known: Flame atomisation Graphite furnace atomisation Mercury hydride atomisation (this is only mentioned here, but not used while doing Standardbase experiments). The source of atoms is usually flame (flame atomisation). Metals could be measured at ppm concentration (part per million, that is mg kg- 1 or mg dm - 3 in case of dilute solutions). The sensitivity could be increased when the light travels for longer in the flame. Therefore most of the burners are about 5-10 cm long. The accuracy is very good, about 1-2%. The sample solution is sprayed (nebulized) continuously into the flame (similarly to the flame photometer). The graphite furnace AAS (GFAAS), a more recent technique is even more sensitive than the traditional, cheaper AAS using flame. Measuremnts could be done at ppb level (part per billion, ppb = 10- 3 ppm, that is g kg- 1 or g dm - 3 in case of dilute solutions!). The accuracy is still about 20% in this latter case. The drying, combustion, vaporisation and atomisation of sample happen in a heated graphite tube that is placed in the way of light. This graphite furnace is protected against oxidation by an inert gas (e.g. argon). The other parts of the atomic absorption spectrophotometer are similar to the one used in the case of UV-Vis spectrophotometer. The monochromator that selects the proper wavelength of the emitted spectra is the usual prism or optical filter. The detector is a photo-electron multiplier that produces an electric sign proportional to the intensity of emitted light. (It contains diodes /electrodes with increasing potential/ between the photocathode and anode, where multiplied
electron emission is caused by the electrons bumping in them.) The electric sign is converted and appears as absorbance on the read-out (fig. 2.)
Fig. 2: A schematic diagram of atomic absorption spectrometer (source: http://www.thebritishmuseum.ac.uk/science/techniques/sr-techaas.html)
The evaluation of ASS measurements is also similar to the UV-Vis spectrophotometry, because it is based on Beers Law, which says that the absorption of light is directly proportional to the number of atoms absorbing it. The more concentrated the sample solution is, the higher absorbance is measured. (For detailed explanation see Standardbase techniques: UV-Vis Spectrophotometry. The same calibration curve or standard addition method described there could be used in case of AAS too. For further information see: http://www.thebritishmuseum.ac.uk/science/techniques/sr-tech-aas.html http://departments.colgate.edu/geology/instruments/aa.htm http://www.arb.ca.gov/aaqm/sop/MLD005_fin.pdf http://www.resonancepub.com/atomicspec.htm Text book available on the Internet: http://las.perkinelmer.com/content/RelatedMaterials/AA%20Concepts%20Book.P DF Reference textbooks: D. A. Skoog - D. M. West - F. J. Holler: Fudamentals of Analytical Chemistry (Saunders College Publishing, Fort Worth, US 1992.)
J. Kenkel: Analytical Chemistry for Technicians (Lewis Publishers, Boca Raton, US 1994.)
Author: Szalay Luca Photograph: Vmos Istvn Institution: Petrik Lajos Vocational School for Chemistry, Environmental Sciences and Information Technology, Budapest, Hungary
You might also like
- Check Maf Kia Carnival DieselDocument7 pagesCheck Maf Kia Carnival Dieseljulio797No ratings yet
- Corrosion Protection of Steel Offshore Units and InstallationsDocument36 pagesCorrosion Protection of Steel Offshore Units and Installationsdamnamyte100% (1)
- Optical Sources, Detectors, and Systems: Fundamentals and ApplicationsFrom EverandOptical Sources, Detectors, and Systems: Fundamentals and ApplicationsNo ratings yet
- Atomic Spectroscopy AnalysisDocument16 pagesAtomic Spectroscopy AnalysisMiftahul JannahNo ratings yet
- Carbohydrate Metabolism Catabolism 2013Document108 pagesCarbohydrate Metabolism Catabolism 2013Anonymous nErkwtXnuS100% (1)
- Atomic AbsorptionDocument27 pagesAtomic Absorptionindustrial technoNo ratings yet
- 02 - STRUCTURE - 01 - European Fitness For Service Network (FITNET) Fatigue Module DevelopmentDocument10 pages02 - STRUCTURE - 01 - European Fitness For Service Network (FITNET) Fatigue Module DevelopmentnotsofarNo ratings yet
- Carbon CompositesDocument30 pagesCarbon CompositesVIbhav GuptaNo ratings yet
- RC Corbel Design (ACI318-05)Document2 pagesRC Corbel Design (ACI318-05)Mohammed Z. AlSaqqa100% (1)
- The WATERMARK CODEX MentalismDocument10 pagesThe WATERMARK CODEX Mentalismtrixter11793100% (1)
- Atomic Absorption SpectrometryDocument36 pagesAtomic Absorption SpectrometryZubair KambohNo ratings yet
- Fundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsFrom EverandFundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in MaterialsRating: 5 out of 5 stars5/5 (1)
- Hacettepe University Department of Chemical Engineering KMÜ 359 Instrumental Analysis Laboratories Experiment 5 Atomic Absorption SpectrosDocument13 pagesHacettepe University Department of Chemical Engineering KMÜ 359 Instrumental Analysis Laboratories Experiment 5 Atomic Absorption SpectrosGamze IdeNo ratings yet
- Atomic Absorption Spectroscopy - SeminarDocument8 pagesAtomic Absorption Spectroscopy - Seminarbolaji4411No ratings yet
- Optical Electronic Spectroscopy 1: Lecture Date: January 23, 2008Document44 pagesOptical Electronic Spectroscopy 1: Lecture Date: January 23, 2008ervaishaliNo ratings yet
- ASAL Biology Sample PDFDocument32 pagesASAL Biology Sample PDFandry hidayatNo ratings yet
- Atomic Absorption Spectroscopy: Basic PrincipleDocument7 pagesAtomic Absorption Spectroscopy: Basic PrincipleSubhecchha BaidyaNo ratings yet
- Atomic Absorption Spectroscopy Review Explains Principles and TechniquesDocument6 pagesAtomic Absorption Spectroscopy Review Explains Principles and TechniquesShajith Ahamed ANo ratings yet
- CHM 312 Note Instrumental-1Document16 pagesCHM 312 Note Instrumental-1makisami62No ratings yet
- Lesson 2: Atomic Absorption Spectroscopy (AAS) : Learning OutcomesDocument14 pagesLesson 2: Atomic Absorption Spectroscopy (AAS) : Learning OutcomesNasima akterNo ratings yet
- AA SpectrosDocument41 pagesAA SpectrosZaryab AliNo ratings yet
- Makalah AAS NovA 300 BingDocument14 pagesMakalah AAS NovA 300 BingAhmad Fadil DjamilNo ratings yet
- Atomic Absorption SpectrosDocument62 pagesAtomic Absorption SpectrosJamal HamadNo ratings yet
- Atomic Absorption and Emission SpectrosDocument10 pagesAtomic Absorption and Emission SpectrosQasim Jalali NanotiNo ratings yet
- Final HUTech FlameDocument4 pagesFinal HUTech FlameNandhanNo ratings yet
- Optical SpectrosDocument7 pagesOptical SpectrosgayaxniNo ratings yet
- AAS TheoryDocument16 pagesAAS Theorygcnayak_blsNo ratings yet
- Atomic Spectroscopy 1Document40 pagesAtomic Spectroscopy 1SOURAV BHATTACHARYANo ratings yet
- Atomic Emission SpectrosDocument14 pagesAtomic Emission SpectrosGjelo CachoNo ratings yet
- Final Aes Lecture NoteDocument6 pagesFinal Aes Lecture NoteEmmanuella OffiongNo ratings yet
- Aes Lecture NoteDocument5 pagesAes Lecture NoteEmmanuella OffiongNo ratings yet
- Atomic Absorption Spectroscopy: Elena SevostianovaDocument7 pagesAtomic Absorption Spectroscopy: Elena Sevostianovaodunmoolorun dorcasNo ratings yet
- Atomic Emission Spectroscopy AsaDocument16 pagesAtomic Emission Spectroscopy AsaMark Cliffton Badlon100% (1)
- Atomic AbsorptionDocument27 pagesAtomic Absorptionindustrial technoNo ratings yet
- Absorption and EmissionDocument15 pagesAbsorption and EmissionFaris AlarshaniNo ratings yet
- Atomic Absorption SpectrosDocument17 pagesAtomic Absorption SpectrosAye Ei MonNo ratings yet
- Atomic SpectrosDocument42 pagesAtomic SpectrosGeleta BekeleNo ratings yet
- 原子激光Document6 pages原子激光li liNo ratings yet
- Atomic SpectraDocument6 pagesAtomic Spectra鄭宇揚No ratings yet
- Atomic SpectrosDocument23 pagesAtomic SpectrosJean Kimberly AgnoNo ratings yet
- Icp Aes PhilipsDocument4 pagesIcp Aes PhilipsAlfonso MartínezNo ratings yet
- Atomic Absorption SpectrometryDocument9 pagesAtomic Absorption SpectrometryRisnaNo ratings yet
- Atomic Mass SpectrosDocument40 pagesAtomic Mass Spectrosjohnpaul varonaNo ratings yet
- Atomic Spectroscopy Sahin EbookDocument11 pagesAtomic Spectroscopy Sahin EbookWW JeonNo ratings yet
- Aas NotesDocument10 pagesAas Notesp.ishaanpawarNo ratings yet
- Lecture 7 (Atomic Absorption Spectroscopy (AAS) ) - ClinicalDocument12 pagesLecture 7 (Atomic Absorption Spectroscopy (AAS) ) - Clinicalayaessam392002No ratings yet
- Determination of Heavy Metal Concentration by Atomic Absorption SpectrosDocument2 pagesDetermination of Heavy Metal Concentration by Atomic Absorption SpectrosNandhini D PNo ratings yet
- What is Atomic Absorption Spectrometry (AASDocument10 pagesWhat is Atomic Absorption Spectrometry (AASabrish fatimaNo ratings yet
- Atomic Absorption SpectrosDocument41 pagesAtomic Absorption SpectrosTasNo ratings yet
- 6 Atomic Spectroscopy 1 0Document22 pages6 Atomic Spectroscopy 1 0os osNo ratings yet
- Flame Spectroscopy (Lecture 6) ClinicalDocument13 pagesFlame Spectroscopy (Lecture 6) Clinicalayaessam392002No ratings yet
- 강의7-Atomic Spect 호환 모드Document39 pages강의7-Atomic Spect 호환 모드Abdoul RahimNo ratings yet
- Atomic Absorption SpectrometryDocument3 pagesAtomic Absorption SpectrometrySean CollinsNo ratings yet
- Atomic Absorption Spectrometry: How It WorksDocument4 pagesAtomic Absorption Spectrometry: How It WorksEhtishamNo ratings yet
- Atomic Absorption SpectrosDocument2 pagesAtomic Absorption SpectrosSoumitra NathNo ratings yet
- Atomic Absorption SpectrosDocument19 pagesAtomic Absorption Spectrosyasir jamilNo ratings yet
- Atomic Spectroscopy: Dong-Sun Lee / Cat - Lab / SWUDocument64 pagesAtomic Spectroscopy: Dong-Sun Lee / Cat - Lab / SWUBalas43100% (1)
- Atomic Absorption Spectroscopy Is A Technique Which Studies Absorption ofDocument8 pagesAtomic Absorption Spectroscopy Is A Technique Which Studies Absorption ofAhmed SaadNo ratings yet
- Atomic Absorption SpectrosDocument5 pagesAtomic Absorption SpectrosEbad Razvi100% (1)
- SAC101 L.12 FlamePhotometer&AASDocument6 pagesSAC101 L.12 FlamePhotometer&AASpradeepkspNo ratings yet
- Determine Elemental Composition Using OESDocument5 pagesDetermine Elemental Composition Using OESmini p shendeNo ratings yet
- Atomic Absorption SpectrosDocument4 pagesAtomic Absorption SpectrosAye Ei MonNo ratings yet
- AasreportDocument28 pagesAasreportBim BoNo ratings yet
- Atomic Emission SpectrometryDocument21 pagesAtomic Emission SpectrometryArslan Muhammad EjazNo ratings yet
- Spectroscopy and Types LectureDocument8 pagesSpectroscopy and Types LectureAnisam AbhiNo ratings yet
- Full Report of Mini ProjectDocument23 pagesFull Report of Mini ProjectNarsyida Niasara HamdanNo ratings yet
- Laser Metrology in Fluid Mechanics: Granulometry, Temperature and Concentration MeasurementsFrom EverandLaser Metrology in Fluid Mechanics: Granulometry, Temperature and Concentration MeasurementsNo ratings yet
- Validation of Raw MaterialDocument22 pagesValidation of Raw MaterialPrima RamadhaniNo ratings yet
- Iso 8502 - 6Document1 pageIso 8502 - 6Ahmad BadriNo ratings yet
- Insulation Resistance Test and Oil Test of Distribution TransformerDocument6 pagesInsulation Resistance Test and Oil Test of Distribution TransformerBash MatNo ratings yet
- Specifyingstainlesssteelsurfacetreatments 10068 PDFDocument5 pagesSpecifyingstainlesssteelsurfacetreatments 10068 PDFshazia khanNo ratings yet
- SPE 37084 Horizontal Well Length: Drill Short or Long Wells?Document9 pagesSPE 37084 Horizontal Well Length: Drill Short or Long Wells?Waleed Barakat MariaNo ratings yet
- JSW Paper On 15Th April, 2008 Technical QuestionsDocument2 pagesJSW Paper On 15Th April, 2008 Technical QuestionsNav ChaNo ratings yet
- Science Test - 1 QuarterDocument3 pagesScience Test - 1 QuarterCeeKay0% (1)
- MZ2000W PDS EnglishDocument3 pagesMZ2000W PDS EnglishLeandroNo ratings yet
- Limit Tests: by Mohneet Chitkara B Pharma Semester 1 2150991058Document13 pagesLimit Tests: by Mohneet Chitkara B Pharma Semester 1 2150991058Mohneet ChitkaraNo ratings yet
- Watson and Crick 1953 Molecular Structure of Nucleic AcidsDocument2 pagesWatson and Crick 1953 Molecular Structure of Nucleic AcidsFabio KochanowskiNo ratings yet
- Exzenter-Membranpumpen Mit Doppeltem Luftdurchsatz VonDocument6 pagesExzenter-Membranpumpen Mit Doppeltem Luftdurchsatz VonSchwarzer Precision GmbH & Co. KGNo ratings yet
- Rules and Refulation For The Classification of Special Service Craft July 2015 - Piping Systems and Presure Plant - ConstructionDocument4 pagesRules and Refulation For The Classification of Special Service Craft July 2015 - Piping Systems and Presure Plant - ConstructionmarboledtNo ratings yet
- Astm C990-03Document4 pagesAstm C990-03Joanne WaiNo ratings yet
- 31 10 00 10-P6000CFP-000-PV - ADocument6 pages31 10 00 10-P6000CFP-000-PV - Aprasenjit pandit100% (1)
- Improved solids flow with hopper insertsDocument4 pagesImproved solids flow with hopper insertsknsaravanaNo ratings yet
- Acticide Bac 50 M MSDS PDFDocument10 pagesActicide Bac 50 M MSDS PDFmeNo ratings yet
- Oxidative WearDocument17 pagesOxidative WearManish Kumar SinghNo ratings yet
- Tablas de Perfiles Estructurales. Beer & Johnston & DeWolf & Mazurek. 6th Edition. 2012 PDFDocument17 pagesTablas de Perfiles Estructurales. Beer & Johnston & DeWolf & Mazurek. 6th Edition. 2012 PDFJGibson FiestasNo ratings yet
- Chapter 6 Final A5 EditDocument16 pagesChapter 6 Final A5 EditkINGNo ratings yet
- BDS Cell StructureDocument66 pagesBDS Cell StructurecheckmateNo ratings yet
- A Finite Element Method Based Analysis of Casting Solidification Onpermanent Metallic ModelsDocument10 pagesA Finite Element Method Based Analysis of Casting Solidification Onpermanent Metallic ModelsseenisitNo ratings yet
- Feasibility of Using Lightweight Eps Based Partitions For Washrooms of ApartmentsDocument6 pagesFeasibility of Using Lightweight Eps Based Partitions For Washrooms of ApartmentsVishnuNo ratings yet