Professional Documents
Culture Documents
Astrazeneca Ab Et. Al. v. Lupin Et. Al.
Uploaded by
PriorSmartOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Astrazeneca Ab Et. Al. v. Lupin Et. Al.
Uploaded by
PriorSmartCopyright:
Available Formats
John E. Flaherty Jonathan M.H.
Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102 (973) 622-4444 Attorneys for Plaintiffs/Counterclaim-Defendants AstraZeneca AB, AstraZeneca LP, KBI-E Inc. and Pozen, Inc. Henry J. Renk Bruce C. Haas Joshua I. Rothman FITZPATRICK, CELLA, HARPER& SCINTO 1290 Avenue of the Americas New York, New York 10104-3800 (212) 218-2100 Of Counsel for Plaintiffs AstraZeneca AB, AstraZeneca LP, and KBI-E Inc.
Stephen M. Hash Stephen C. Stout Deirdre Dorval Shannon Kidd VINSON & ELKINS LLP 2801 Via Fortuna, Suite 100 Austin, TX 78746-7568 (512) 542-8400 Of Counsel for Plaintiff Pozen Inc. Einar Stole Edward H. Rippey Maureen M. Japha COVINGTON & BURLING LLP 1201 Pennsylvania Avenue, NW Washington, DC 20004-2401 (202) 662-6000 Of Counsel for Plaintiffs AstraZeneca AB, AstraZeneca LP, and KBI-E Inc.
UNITED STATES DISTRICT COURT FOR THE DISTRICT OF NEW JERSEY ASTRAZENECA AB, ASTRAZENECA LP, KBI-E INC., and POZEN, INC., Plaintiffs, v. LUPIN LTD. and LUPIN PHARMACEUTICALS INC., Defendants. COMPLAINT FOR PATENT INFRINGEMENT AND CERTIFICATION PURSUANT TO LOCAL CIVIL RULE 11. Civil Action No.
Plaintiffs AstraZeneca AB, AstraZeneca LP, KBI-E Inc., and Pozen Inc. (collectively, Plaintiffs), by their attorneys, for their Complaint against Defendants Lupin Ltd. and Lupin Pharmaceuticals Inc. (collectively, Defendants), allege as follows: NATURE OF THE ACTION 1. This is a civil action for patent infringement arising under the patent laws of the
United States, 35 U.S.C. 100 et seq., and in particular under 35 U.S.C. 271(e). This action relates to Abbreviated New Drug Application (ANDA) No. 202654 filed by or for the benefit of Defendants with the United States Food and Drug Administration (FDA) for approval to market generic versions of Plaintiffs VIMOVO pharmaceutical products that are sold in the United States. THE PARTIES 2. Plaintiff AstraZeneca AB (AZ AB) is a corporation operating and existing
under the laws of Sweden, with its principal place of business at S-151 85 Sdertlje, Sweden. 3. Plaintiff AstraZeneca LP (AZ LP) is a limited partnership operating and
existing under the laws of the State of Delaware, with its principal place of business at 1800 Concord Pike, Wilmington, Delaware 19803. 4. Plaintiff KBI-E Inc. (KBI-E) is a corporation operating and existing under the
laws of the State of Delaware, with its principal place of business in Wilmington, Delaware. 5. Plaintiff Pozen Inc. (Pozen) is a corporation operating and existing under the
laws of the State of Delaware, with its principal place of business at 1414 Raleigh Road, Chapel Hill, North Carolina 27517. 6. On information and belief, Defendant Lupin Ltd. (Lupin Ltd.) is a corporation
operating and existing under the laws of India, with its principal place of business at B/4 Laxmi 2
Towers, Bandra Kurla Complex, Bandra (E), Mumbai 400 051, India, and its registered office at 159 CST Road, Kalina, Santacruz (E), Mumbai 400 098, India. 7. On information and belief, Defendant Lupin Pharmaceuticals Inc. (Lupin Inc.)
is a corporation operating and existing under the laws of the Commonwealth of Virginia, with its principal place of business at 111 South Calvert Street 21st Floor, Baltimore, MD 21202. 8. On information and belief, Lupin Inc. is a wholly-owned subsidiary of Lupin Ltd. BACKGROUND The NDA 9. AZ LP is the holder of New Drug Application (NDA) No. 022511 for
VIMOVO (naproxen and esomeprazole magnesium) Delayed Release Tablets, in 375 mg (naproxen)/20 mg (esomeprazole magnesium) and 500 mg (naproxen)/20 mg (esomeprazole magnesium) dosage forms. 10. VIMOVO is a prescription drug approved for use to relieve the signs and
symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, and to decrease the risk of stomach (gastric) ulcers in patients at risk of developing stomach ulcers from treatment with non-steroidal anti-inflammatory drugs (NSAIDs). Naproxen and esomeprazole magnesium are the active ingredients in VIMOVO. The Patent-In-Suit 11. United States Patent No. 8,557,285 (the 285 patent), entitled Pharmaceutical
Compositions for the Coordinated Delivery of NSAIDs, was duly and legally issued by the United States Patent and Trademark Office on October 15, 2013. The claims of the 285 patent
are directed to pharmaceutical compositions in unit dosage form comprising esomeprazole and naproxen. A true and correct copy of the 285 patent is attached as Exhibit A. 12. Pozen owns the 285 patent by assignment from the inventor John R. Plachetka.
AZ AB is Pozens exclusive licensee under the 285 patent. The 285 patent will expire on May 31, 2022. 13. The 285 patent is a patent with respect to which a claim of patent infringement
could reasonably be asserted if a person not licensed by the owner engaged in the manufacture, use, or sale of the VIMOVO drug product. 14. Accordingly, pursuant to 21 U.S.C. 355(c)(2), Pozen and the AstraZeneca
Plaintiffs are submitting patent information for the 285 patent to the FDA in connection with their NDA No. 022511 for VIMOVO drug product. The FDA is expected to publish the same in the Orange Book. The ANDA 15. On information and belief, Lupin Ltd. filed ANDA No. 202654 with the FDA
under 21 U.S.C. 355(j) to obtain FDA approval for Lupin Ltd. and Lupin Inc. to commercially manufacture, use, import, offer for sale, and sell in the United States naproxen and esomeprazole magnesium delayed release tablets containing 375 mg (naproxen)/20 mg (esomeprazole magnesium) or 500 mg naproxen (naproxen)/20 mg (esomeprazole magnesium) (Lupins Naproxen and Esomeprazole Magnesium Delayed Release Tablets or the ANDA Products), which are generic versions of Plaintiffs VIMOVO Delayed Release Tablets in 375 mg (naproxen)/20 mg (esomeprazole magnesium) and 500 mg (naproxen)/20 mg (esomeprazole magnesium) strengths, respectively.
16.
By letter dated June 10, 2011 (the ANDA Notice Letter), Lupin Ltd. notified
Plaintiffs that it had filed ANDA No. 202654 seeking approval to market Lupins Naproxen and Esomeprazole Magnesium Delayed Release Tablets and was providing information to Plaintiffs pursuant to 21 U.S.C. 355(j)(2)(B)(ii) and 21 C.F.R. 314.95. JURISDICTION AND VENUE 17. Subject matter jurisdiction over this action is proper pursuant to the provisions of
Title 28, United States Code, Sections 1331 and 1338(a). 18. On information and belief, Defendants are in the business of developing,
formulating, manufacturing, marketing, offering to sell, selling and commercializing pharmaceutical products. 19. On information and belief, Lupin Ltd., either directly or through one or more of
its wholly owned subsidiaries and/or agents, develops, manufactures, distributes, markets, offers to sell, and sells generic drug products for sale and use throughout the United States, including within this judicial district. 20. On information and belief, Lupin Inc., with the assistance and/or at the direction
of Lupin Ltd., develops, manufactures, distributes, markets, offers to sell, and sells generic drug products for sale and use throughout the United States, including within this judicial district. 21. On information and belief, Defendants acted in concert to develop Lupins
Naproxen and Esomeprazole Magnesium Delayed Release Tablets, and to seek approval from the FDA to sell Lupins Naproxen and Esomeprazole Magnesium Delayed Release Tablets throughout the United States, including within this judicial district. 22. On information and belief, both Lupin Ltd. and Lupin Inc. have been and are
engaging in activities directed toward infringement of the 285 patent (the patent-in-suit) by, 5
inter alia, preparing and/or submitting ANDA No. 202654 seeking FDA approval to market Lupins Naproxen and Esomeprazole Magnesium Delayed Release Tablets. As stated in the ANDA Notice Letter, Defendants intend to market Lupins Naproxen and Esomeprazole Magnesium Delayed Release Tablets before expiration of the patent-in-suit. On information and belief and as stated in the ANDA Notice Letter, the FDA received ANDA No. 202654 from Lupin Ltd. 23. In its ANDA Notice Letter, Lupin Ltd. stated that the name and address of its
agent in the United States authorized to accept service of process for Defendants for purposes of an infringement action based upon its ANDA Notice Letter is Robert F. Green of Leydig, Voit and Mayer Ltd., 180 North Stetson, Suite 4900, Chicago, IL 60601. 24. Upon information and belief, Lupin Ltd. is subject to personal jurisdiction in New
Jersey because, among other things, Lupin Ltd., itself and through its wholly owned subsidiary Lupin Inc., has purposely availed itself of the benefits and protections of New Jerseys laws such that it should reasonably anticipate being haled into court in New Jersey. Upon information and belief, Lupin Ltd., itself and through its wholly owned subsidiary Lupin Inc., manufactures, markets, and/or sells generic drugs throughout the United States and within the State of New Jersey, and therefore transacts business within the State of New Jersey related to Plaintiffs claims, and/or has engaged in systematic and continuous business contacts within the State of New Jersey. Lupin Ltd. is subject to personal jurisdiction in New Jersey on the basis of its inducement of and/or contribution to Lupin Inc.s acts of infringement in New Jersey. In addition, Lupin Ltd. is subject to personal jurisdiction in New Jersey because, on information and belief, it controls and dominates Lupin Inc. and therefore the activities of Lupin Inc. in this jurisdiction are attributed to Lupin Ltd.
25.
On information and belief, this Court has personal jurisdiction over Lupin Inc.
because Lupin Inc. has purposely availed itself of the benefits and protections of New Jerseys laws such that it should reasonably anticipate being haled into court in New Jersey. Upon information and belief, Lupin Inc. manufactures, markets, and/or sells generic drugs throughout the United States and within the State of New Jersey and therefore transacts business within the State of New Jersey related to Plaintiffs claims, and/or has engaged in systematic and continuous business contacts within the State of New Jersey. 26. On information and belief, Lupin Inc. is registered to do business in New Jersey
(business identification number 0100953673) and has appointed National Registered Agents, Inc., located at 100 Canal Pointe Blvd., Suite 212, Princeton, NJ 08540, as its registered agent for the receipt of service of process. 27. On information and belief, both Lupin Ltd. and Lupin Inc. have previously been
sued in this district and have not challenged personal jurisdiction. See, e.g., AstraZeneca AB et al. v. Lupin Ltd. et al, Civ. Action No. 3:11-cv-04275-JAP-DEA (D.N.J.); AstraZeneca AB et al. v. Lupin Ltd. and Lupin Pharm. Inc., Civ. Action No. 3:09-cv-05404-JAP-TJB (D.N.J.); Abbott Labs and Laboratoires Fournier S.A. v. Lupin Ltd. and Lupin Pharm. Inc., Civ. Action No. 2:09cv-01007-GEB-MCA (D.N.J.); Abbott Labs and Laboratoires Fournier S.A. v. Lupin Ltd. and Lupin Pharm. Inc., Civ. Action No. 2:10-cv-01578-DMC-JAD (D.N.J.); Tibotec Inc. and Tibotec Pharm. v. Lupin Ltd., et al., Civ. Action No. 2:10-cv-05954-WHW-MAS (D.N.J.); Novartis Corp. et al. v. Lupin Ltd. and Lupin Pharm. Inc., Civ. Action No. 2:06-cv-05954-GEB-ES (D.N.J.); and Elan Intl. Ltd. and Fournier Laboratories Ireland Ltd., Civ. Action No. 2:09-cv01008-GEB-MCA (D.N.J.).
28.
On information and belief, Lupin Ltd. and Lupin Inc. have availed themselves of
the jurisdiction of this court by initiating litigation in this district. See, e.g., Lupin Ltd. and Lupin Pharm. Inc. v. Merck, Sharp & Dohme Corp., Civ. Action No. 3:10-CV-683-JAP-TJB (D.N.J.). 29. On information and belief, by virtue of, inter alia, Defendants continuous and
systematic contacts with New Jersey, including but not limited to the above-described contacts, and the actions on behalf of Defendants in connection with ANDA No. 202654, this Court has personal jurisdiction over Defendants. These activities satisfy due process and confer personal jurisdiction over Defendants consistent with New Jersey law. 30. Venue is proper in this District pursuant to the provisions of Title 28, United
States Code, Sections 1391(c) and (d), and 1400(b). COUNT I (INFRINGEMENT OF THE 285 PATENT UNDER 35 U.S.C. 271(e)(2)(A)) 31. Plaintiffs incorporate by reference paragraphs 1-28 of this Complaint as if fully
set forth herein. 32. On information and belief, the making, using, selling, and/or offering for sale in
the United States of Defendants pharmaceutical compositions in unit dosage form comprising esomeprazole and naproxen described in Defendants ANDA infringes the 285 patent. 33. Defendants have infringed the 285 patent under 35 U.S.C. 271 (e)(2) by filing
their ANDA and continuing to seek approval from the FDA to engage in the commercial manufacture, use, or sale of a drug claimed in this patent, prior to the expiration of the 285 patent. 34. On information and belief, the ANDA Products contain the pharmaceutical
composition patented in the 285 patent, constitute a material part of the inventions of the 285 patent, are especially made or especially adapted for use in an infringement of the 285 patent, 8
and are not a staple article or commodity of commerce suitable for substantial noninfringing use. Upon information and belief, Defendants are aware that the ANDA Products are so made or so adapted. Upon information and belief, Defendants are aware that the ANDA Products, if
approved, will be used in contravention of Plaintiffs rights under the 285 patent. 35. On information and belief, Defendants have previously filed patent certifications
in association with their ANDA No. 202654 seeking, inter alia, FDA final approval prior to November 27, 2014. The 285 patent has an expiration date of May 31, 2022. Therefore, on further information and belief, Defendants are currently pursuing FDA final approval of their ANDA No. 202654 prior to the expiration date of the 285 patent. 36. Pursuant to 21 U.S.C. 355(j)(2)(A)(vii), Defendants should file a patent
certification in thier pending ANDA No. 202654 with respect to the 285 patent, and Defendants must make a Paragraph IV Certification with respect to the 285 patent if Defendants continue to seek FDA final approval of their ANDA No. 202654 prior to May 31, 2022. On information and belief, Defendants above-described activities are continuing and constitute an act of infringement of the 285 Patent under 35 U.S.C. 271(e)(2). 37. On information and belief, the manufacture, use, and sale of the ANDA Products
infringes the 285 patent claims. 38. Plaintiffs will be substantially and irreparably harmed by the infringing activities
described above unless those activities are precluded by this Court. Plaintiffs have no adequate remedy at law. PRAYER FOR RELIEF WHEREFORE, Plaintiffs respectfully request the following relief: A. A judgment that the claims of the patent-in-suit are valid and enforceable; 9
B.
A judgment that the submission of ANDA No. 202654 by Defendants infringes
one or more claims of the patent-in-suit under 35 U.S.C. 271(e)(2)(A); C. A judgment providing that, pursuant to 35 U.S.C. 271(e)(4)(A), the effective
date of any FDA approval of Defendants ANDA No. 202654 shall be no earlier than the later of the expiration date of the patent-in-suit or any later exclusivity to which Plaintiffs are or become entitled; D. A judgment pursuant to 35 U.S.C. 271(e)(4)(B) permanently enjoining
Defendants, and all persons acting in concert with any of them, from making, using, selling, offering to sell, or importing the naproxen and esomeprazole magnesium product described in Defendants ANDA No. 202654 no earlier than the later of the expiration date of the patent-insuit or any later exclusivity to which Plaintiffs are or become entitled; E. F. G. Attorneys fees in this action pursuant to 35 U.S.C. 285; Costs and expenses in this action; and Such further and other relief as this Court may deem just and proper.
10
Dated: October 23, 2013
Respectfully Submitted, By: s/ John E. Flaherty John E. Flaherty Jonathan M.H. Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102 (973) 622-4444 Attorneys for Plaintiffs ASTRAZENECA AB, ASTRAZENECA LP, POZEN, INC. and KBI-E INC. Of Counsel: Henry J. Renk Bruce C. Haas Joshua I. Rothman FITZPATRICK, CELLA, HARPER & SCINTO 1290 Avenue of the Americas New York, New York 10104-3800 (212) 218-2100 Einar Stole Edward H. Rippey Maureen M. Japha COVINGTON & BURLING LLP 1201 Pennsylvania Avenue, N.W. Washington, D.C. 20004-2401 (202) 662-6000 Stephen M. Hash Stephen C. Stout Deirdre Dorval Shannon Kidd VINSON & ELKINS LLP 2801 Via Fortuna, Suite 100 Austin, TX 78746-7568 (512) 542-8400
11
CERTIFICATION PURSUANT TO L. CIV. R. 11.2 Pursuant to Local Civil Rule 11.2, I hereby certify that the matter in controversy is the subject of the following actions: ASTRAZENECA AB et al. v. DR. REDDYS LABS. INC., et al., C.A. No. 3:11-cv-02317JAP-DEA (D.N.J.); ASTRAZENECA AB et al. v. DR. REDDYS LABS. INC. et al, C.A. No. 3:13-cv-00091JAP-DEA (D.N.J.); ASTRAZENECA AB et al. v. LUPIN LTD., et al., C.A. No. 3:11-cv-04275-JAP-DEA (D.N.J.); ASTRAZENECA AB et al. v. ANCHEN PHARMS., INC., C.A. No. 3:11-cv-06348-JAPDEA (D.N.J.); ASTRAZENECA AB et al. v. WATSON LABORATORIES, INC.- FLORIDA, et al., C. A. No. 3:13-cv-03038-JAP-DEA (D.N.J.); ASTRAZENECA AB et al. v. MYLAN PHARMACEUTICALS et al., C.A. No. 3:13-cv04022-JAP-DEA (D.N.J.) ASTRAZENECA AB, et al. v. MYLAN LABORATORIES LTD. et al., C.A. No. 3:12-cv01378-JAP-TJB (D.N.J.); ASTRAZENECA AB et al. v. WATSON LABORATORIES, INC. - FLORIDA et al., C.A. No. 3:13-cv-01669-JAP-TJB (D.N.J.); and ASTRAZENECA AB et al. v. WOCKHARDT LIMITED et al., C.A. No. 3:13-cv-04854JAP-TJB (D.N.J.) The foregoing cases involve products that contain an esomeprazole magnesium formulation. The matter in controversy involves the same esomeprazole magnesium formulations. All of these cases have been assigned to Hon. Joel A. Pisano, U.S.D.J. The DRL, Lupin, and Anchen cases have been consolidated for discovery purposes and have been assigned to Magistrate Judge Arpert. Therefore, for the sake of judicial economy and with regard to Judge Pisanos and Judge Arperts familiarity of the patents asserted in the matter in controversy, Plaintiffs believe these 12
cases and the matter in controversy are all related. Accordingly, Plaintiffs respectfully request that the matter in controversy be assigned to Judge Pisano and Magistrate Judge Arpert.
13
Dated: October 23, 2013
Respectfully Submitted, By: s/ John E. Flaherty John E. Flaherty Jonathan M.H. Short McCARTER & ENGLISH, LLP Four Gateway Center 100 Mulberry Street Newark, New Jersey 07102 (973) 622-4444 Attorneys for Plaintiffs ASTRAZENECA AB, ASTRAZENECA LP, KBI-E INC, And Pozen, Inc. Of Counsel: Henry J. Renk Bruce C. Haas Joshua I. Rothman FITZPATRICK, CELLA, HARPER & SCINTO 1290 Avenue of the Americas New York, New York 10104-3800 (212) 218-2100 Einar Stole Edward H. Rippey Maureen M. Japha COVINGTON & BURLING LLP 1201 Pennsylvania Avenue, N.W. Washington, D.C. 20004-2401 (202) 662-6000 Stephen M. Hash Stephen C. Stout Deirdre Dorval Shannon Kidd VINSON & ELKINS LLP 2801 Via Fortuna, Suite 100 Austin, TX 78746-7568 (512) 542-8400
14
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Free Qigong EbookDocument33 pagesFree Qigong Ebookpenntara90% (10)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Serrapeptase - The Miracle Enzyme Which Can Save Your LifeDocument53 pagesSerrapeptase - The Miracle Enzyme Which Can Save Your LifeRoger-Peter Weizenegger100% (7)
- Techniques in Family TherapyDocument23 pagesTechniques in Family TherapyAntónio Martins100% (4)
- Peripheral Vascular DiseaseDocument53 pagesPeripheral Vascular DiseaseShenbagam Mahalingam100% (1)
- Assessment Needs Nursing Diagnos IS Goal/Obj Ective Intervention Rationale EvaluationDocument10 pagesAssessment Needs Nursing Diagnos IS Goal/Obj Ective Intervention Rationale EvaluationApol Pen67% (3)
- Wilhelm Reich Contact With SpaceDocument6 pagesWilhelm Reich Contact With Spacebubbaclem100% (2)
- Diabetes Management: OverviewDocument20 pagesDiabetes Management: Overviewlaloo01No ratings yet
- Doctor's OrdersDocument6 pagesDoctor's OrdersKryza Dale Bunado BaticanNo ratings yet
- Final Na Talaga ToDocument54 pagesFinal Na Talaga ToJoy ManguneNo ratings yet
- NCP For Laryngeal CancerDocument5 pagesNCP For Laryngeal CancerMădălina PinciucNo ratings yet
- Quick Reference Guide-04Dec2019Document46 pagesQuick Reference Guide-04Dec2019adiangriawanNo ratings yet
- Like Kind Card Game (US Patent 6193235)Document12 pagesLike Kind Card Game (US Patent 6193235)PriorSmartNo ratings yet
- Multicasting Method and Apparatus (US Patent 6434622)Document46 pagesMulticasting Method and Apparatus (US Patent 6434622)PriorSmartNo ratings yet
- Like Kind Money Board Table Game (US Patent 6186505)Document11 pagesLike Kind Money Board Table Game (US Patent 6186505)PriorSmartNo ratings yet
- Intelligent User Interface Including A Touch Sensor Device (US Patent 8288952)Document9 pagesIntelligent User Interface Including A Touch Sensor Device (US Patent 8288952)PriorSmartNo ratings yet
- Cell Regulatory Genes, Encoded Products, and Uses Related Thereto (US Patent 7030227)Document129 pagesCell Regulatory Genes, Encoded Products, and Uses Related Thereto (US Patent 7030227)PriorSmartNo ratings yet
- Method and Apparatus For Retrieving Data From A Network Using Linked Location Identifiers (US Patent 6226655)Document22 pagesMethod and Apparatus For Retrieving Data From A Network Using Linked Location Identifiers (US Patent 6226655)PriorSmartNo ratings yet
- User Interface With Proximity Sensing (US Patent 8035623)Document15 pagesUser Interface With Proximity Sensing (US Patent 8035623)PriorSmartNo ratings yet
- Wine Cellar Alarm System (US Patent 8710985)Document11 pagesWine Cellar Alarm System (US Patent 8710985)PriorSmartNo ratings yet
- Advance Products & Systems v. CCI Piping SystemsDocument5 pagesAdvance Products & Systems v. CCI Piping SystemsPriorSmartNo ratings yet
- Casing Spacer (US Patent 6736166)Document10 pagesCasing Spacer (US Patent 6736166)PriorSmartNo ratings yet
- High-Speed Serial Linking Device With De-Emphasis Function and The Method Thereof (US Patent 7313187)Document10 pagesHigh-Speed Serial Linking Device With De-Emphasis Function and The Method Thereof (US Patent 7313187)PriorSmartNo ratings yet
- Casino Bonus Game Using Player Strategy (US Patent 6645071)Document3 pagesCasino Bonus Game Using Player Strategy (US Patent 6645071)PriorSmartNo ratings yet
- TracBeam v. AppleDocument8 pagesTracBeam v. ApplePriorSmartNo ratings yet
- Modern Telecom Systems LLCDocument19 pagesModern Telecom Systems LLCPriorSmartNo ratings yet
- Eckart v. Silberline ManufacturingDocument5 pagesEckart v. Silberline ManufacturingPriorSmartNo ratings yet
- Senju Pharmaceutical Et. Al. v. Metrics Et. Al.Document12 pagesSenju Pharmaceutical Et. Al. v. Metrics Et. Al.PriorSmartNo ratings yet
- VIA Technologies Et. Al. v. ASUS Computer International Et. Al.Document18 pagesVIA Technologies Et. Al. v. ASUS Computer International Et. Al.PriorSmartNo ratings yet
- Richmond v. Creative IndustriesDocument17 pagesRichmond v. Creative IndustriesPriorSmartNo ratings yet
- Senju Pharmaceutical Et. Al. v. Metrics Et. Al.Document12 pagesSenju Pharmaceutical Et. Al. v. Metrics Et. Al.PriorSmartNo ratings yet
- Perrie v. PerrieDocument18 pagesPerrie v. PerriePriorSmartNo ratings yet
- ATEN International v. Uniclass Technology Et. Al.Document14 pagesATEN International v. Uniclass Technology Et. Al.PriorSmartNo ratings yet
- Sun Zapper v. Devroy Et. Al.Document13 pagesSun Zapper v. Devroy Et. Al.PriorSmartNo ratings yet
- Merck Sharp & Dohme v. Fresenius KabiDocument11 pagesMerck Sharp & Dohme v. Fresenius KabiPriorSmartNo ratings yet
- Mcs Industries v. Hds TradingDocument5 pagesMcs Industries v. Hds TradingPriorSmartNo ratings yet
- Merck Sharp & Dohme v. Fresenius KabiDocument10 pagesMerck Sharp & Dohme v. Fresenius KabiPriorSmartNo ratings yet
- TracBeam v. T-Mobile Et. Al.Document9 pagesTracBeam v. T-Mobile Et. Al.PriorSmartNo ratings yet
- GRQ Investment Management v. Financial Engines Et. Al.Document12 pagesGRQ Investment Management v. Financial Engines Et. Al.PriorSmartNo ratings yet
- Dok Solution v. FKA Distributung Et. Al.Document99 pagesDok Solution v. FKA Distributung Et. Al.PriorSmartNo ratings yet
- Multiplayer Network Innovations v. Konami Digital EntertainmentDocument6 pagesMultiplayer Network Innovations v. Konami Digital EntertainmentPriorSmartNo ratings yet
- Shenzhen Liown Electronics v. Luminara Worldwide Et. Al.Document10 pagesShenzhen Liown Electronics v. Luminara Worldwide Et. Al.PriorSmartNo ratings yet
- Kyasanur Forest DiseaseDocument2 pagesKyasanur Forest DiseaseGenoMacaraanNo ratings yet
- DHN 374 News BriefDocument2 pagesDHN 374 News Briefapi-340516995No ratings yet
- An Exploratory BriefDocument11 pagesAn Exploratory BriefElizabeth Roxan Lizaso GonzálezNo ratings yet
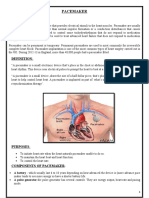
- PACEMAKERDocument5 pagesPACEMAKERPoonam ThakurNo ratings yet
- BHU Daily Stock PerformaDocument6 pagesBHU Daily Stock PerformabilalNo ratings yet
- AradaleDocument8 pagesAradaleCharlotteNo ratings yet
- Case Presentation and Discussion On Chronic Obstructive Pulmonary DiseaseDocument15 pagesCase Presentation and Discussion On Chronic Obstructive Pulmonary DiseaseCalingalan Hussin CaluangNo ratings yet
- Workshop 0708Document3 pagesWorkshop 0708Manojkumar NairNo ratings yet
- LMA Talk Kelvin 2010 AGDocument26 pagesLMA Talk Kelvin 2010 AGGbotemi AlaladeNo ratings yet
- Jpoa 2023 Feb PDFDocument163 pagesJpoa 2023 Feb PDFOscar MontilvaNo ratings yet
- Leg CellulitisDocument3 pagesLeg CellulitisJessica Pacris MaramagNo ratings yet
- Ethics and Patient SafetyDocument10 pagesEthics and Patient SafetyIdeas InfiniteNo ratings yet
- Narrative Therapy Tree of Life ProjectDocument2 pagesNarrative Therapy Tree of Life ProjecttduongNo ratings yet
- Preguntas Sobre Varios TemasDocument4 pagesPreguntas Sobre Varios TemasGerardo Davalos GuzmanNo ratings yet
- Biography of Dorothea OremDocument6 pagesBiography of Dorothea OremGeorgeEchevarriaNo ratings yet
- Venipuncture (Procedure) : Patrick Kyle C. Ignacio, RRTDocument26 pagesVenipuncture (Procedure) : Patrick Kyle C. Ignacio, RRTCielo DeguzmanNo ratings yet
- Koe V Noggle Injunction OrderDocument83 pagesKoe V Noggle Injunction OrderLindsey BasyeNo ratings yet
- Keratosis Pilaris Rubra and Keratosis PiDocument5 pagesKeratosis Pilaris Rubra and Keratosis Pidaily of sinta fuNo ratings yet
- Hernia+scrotal MassDocument82 pagesHernia+scrotal MassJJ JirapathNo ratings yet