Professional Documents
Culture Documents
Protein Interactors of The Cellular Tumor Antigen p53 With Response To Ionizing Radiation
Uploaded by
Roberto PinedaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Protein Interactors of The Cellular Tumor Antigen p53 With Response To Ionizing Radiation
Uploaded by
Roberto PinedaCopyright:
Available Formats
IPEN
Informe Cientfico Tecnolgico 2012. p. 49-50. ISSN 1684-1662
Protein interactors of the cellular tumor antigen p53 with response to ionizing radiation
Silvia Vsquez1,3,4,*, Roberto Pineda2, Mohanalatha Chandrasekharan3, Prashanth Suravajhala3,4
1
Direccin de Investigacin y Desarrollo, Centro Nuclear RACSO, Instituto Peruano de Energa Nuclear, Av. Canad 1470, Lima 41, Per 2 Facultad de Ciencias Biolgicas, Universidad Ricardo Palma, Av. Benavides 5440, Surco, Lima 33, Per 3 Bioclues.org, IKP Knowledge Park, Secunderabad, 500009 AP, India 4 Bioinformatics Organization, Hudson, Massachusetts, United States of America
Abstract
TP53 interacts with the genes CDKN1A, CSNK1A1, CSNK1D, BAX, ATM, MDM2, HIF1A,SP1. This consensus network has many binding regions involved in phosphorylation. To find whether or not the interactions are involved in ionizing radiation (IR), we used protein interaction mining and visualization tools to decipher the network. We conclude that
ATM, PCNA, TP53 and CDKN1A show protein interactions on cellular response to IR. Resumen
TP53 interacta con los genes CDKN1A, CSNK1A1, CSNK1D, BAX, ATM, MDM2, HIF1A y SP1. La red consensual mencionada presenta varias regiones de unin que intervienen durante la fosforilacin. Para encontrar si estas interacciones participan o no en la respuesta a la radiacin ionizante (IR) analizamos la minera de interacciones proteicas y las herramientas de visualizacin para descifrar la red. Concluimos que ATM, PCNA, TP53 y CDKN1A presentan interacciones proteicas en la respuesta celular a IR.
1.
Introduction
The cellular tumor antigen p53[1] participates in the regulation of the cellular cycle and the tumor suppression as a cellular response. The gene TP53 is located on the chromosome 17 (17p13.1) in humans with the encoded protein for 393 amino acids and four units or domains where one domain activates the transcription factors while another recognizes specific DNA sequences. Together they are called the core domains and are responsible for the protein tetramerization recognizing the DNA damage. Earlier, we had explored the role of p53 in cardiovascular diseases CVD [2]. Our erstwhile approach, we believe has narrowed down the experimentation in finding better candidates for p53 in cardiovascular system. While MSH2 is known to be found in both mitochondria and cytoplasm (or nucleus), the subcellular location studies we had employed has given a validation that MSH2 might also play an important role in deciphering the role of these proteins linked to p53, towards CVD. We believe and envisage that the other
proteins could also be better candidates and it would be interesting to run pull down assays for these proteins to check for their candidacy in CVD. With TP53 interacting with host of genes, viz. CDKN1A, CSNK1A1, CSNK1D, BAX, ATM, MDM2, HIF1A, SP1, a consensus protein interaction networks of physical interactions need to be built which would allow us to find genes involved in phosphorylation of Thr-145 by Akt or of Ser146. These impair binding to PCNA while phosphorylation at Ser-114 by GSK3-beta enhances ubiquitination by the DCX (DTL) complex. Phosphorylation of Thr-145 by PIM2 is known to enhance CDKN1A stability and inhibits cell proliferation. Whereas phosphorylation of Thr-145 by PIM1 results in the relocation of CDKN1A to the cytoplasm and enhanced CDKN1A protein stability, ubiquitination by MKRN1 leads to polyubiquitination and 26S proteasome-dependent degradation.*
Corresponding author: svasquez@ipen.gob.pe
49
IPEN
Informe Cientfico Tecnolgico 2012. p. 49-50. ISSN 1684-1662
2.
Methods
Through this study, we exploited the protein interaction partners by showing evidence of interactions for ATM and CDKN1A, known to be involved in cellular response to IR [3]. We used protein interaction mining and visualization tools in the form of Genecards [4], Genemania [5] and String [6] to bring out the interactions and analyze them towards radiosensitivity studies. The methods discussed can be linked to our previous studies [2].
double strand breaks (DSBs) thereby regulating DNA damage response mechanism. Also known to be involved in signal transduction and cell cycle control, the gene plays a very important role as a tumor suppressor.
4.
Conclusion
There is a lacunae of PPI studies involving cardiovasuclar diseased genes. We aimed to delve into these PPI studies as a whole allow to find the interacting genes involved in participation of cellular repertoire after the IR. Our analysis found ATM, PCNA, TP53 and CDNK1A as evident interactors related to IR. We now aim to identify genes and proteins participating at different levels of the cellular systems and validate the approaches in the wet lab.
5.
References
[1] PO4637 (Reviewed entry), UniProtKB /Swiss-Prot. Database. UniProtKB, Last modified May 29; 2013. Versin 209. Access date Jun. 10. 2013.
http://www.uniprot.org/uniprot/P04637
[2] Chandrasekharan M, Vasquez S, Galimudi RK, Suravajhala P. Toward understanding the role of p53 in cardiovascular diseases. J. Biomedical Science and Engineering. 2013; 6:209-212. [3] Pernot E, Hall J, et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutation Research. 2012; 751:258-286. [4] The GeneCards human gene database, Genecards, Last modified Apr 28; 2013. Versin 3.10, Access date Jun. 15. 2013.
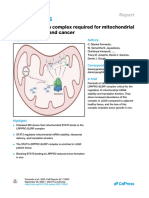
Figure 1. ATM, PCNA, TP53 and CDNK1A in a cloud of interactome.
3.
Results and Discussion
We reviewed that CDNK1A is an important intermediate by which p53 mediates its role as an inhibitor of cellular proliferation in response to DNA damage. Further, it binds to and inhibits cyclin-dependent kinase activity, preventing phosphorylation of critical cyclindependent kinase substrates and blocking cell cycle progression while ataxia telangiectasia mutated (ATM) is a serine/threonine protein kinase involved in activatation of checkpoint signaling upon double strand breaks (DSBs), apoptosis and genotoxic stresses (Figure 1). This allows the gene act as a DNA damage sensor. Further, it recognizes the substrate consensus sequence [ST]-Q phosphorylates 'Ser-139' of histone variant H2AX/H2AFX at
http://www.genecards.org
[5] GeneMANIA networks, GeneMANIA, Last modified Jul 19; 2012. Version 3.1.2, Access date Jun.15. 2013.
http://www.genemania.org
[6] Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013 Jan; 41(Database issue):D808-15.
50
You might also like
- p53-Regulated lncRNA Activates Enhancer DomainsDocument12 pagesp53-Regulated lncRNA Activates Enhancer DomainskNo ratings yet
- Research Paper On Protein BindingDocument7 pagesResearch Paper On Protein Bindingnadevufatuz2100% (1)
- A STAT3 Protein Complex Required For Mitochondrial mRNA Stability and CancerDocument15 pagesA STAT3 Protein Complex Required For Mitochondrial mRNA Stability and CancerAna MorelliNo ratings yet
- ATEROESCLEROSISDocument228 pagesATEROESCLEROSISTenazNo ratings yet
- Art:10.1007/s00401 013 1088 7Document11 pagesArt:10.1007/s00401 013 1088 7TT WeiNo ratings yet
- Dkk1 mediates MPP+-induced neurotoxicity in PC12 cellsDocument8 pagesDkk1 mediates MPP+-induced neurotoxicity in PC12 cellsShawnNo ratings yet
- Tmpe615 TMPDocument8 pagesTmpe615 TMPFrontiersNo ratings yet
- Srinivasan 2016Document16 pagesSrinivasan 2016Zeljko TomljanovicNo ratings yet
- 2 Structure: Tumor Protein p53, Also Known As p53, Cellular p53 (p53, Tumor Suppressor p53, Antigen NY-CO-13Document21 pages2 Structure: Tumor Protein p53, Also Known As p53, Cellular p53 (p53, Tumor Suppressor p53, Antigen NY-CO-13ZiedTrikiNo ratings yet
- Antioxidants 11 00726 v3Document16 pagesAntioxidants 11 00726 v3boopathiraja.chinnasamyNo ratings yet
- Modification of Drosophila p53 by SUMO Modulates Its Transactivation and Pro-Apoptotic FunctionsDocument9 pagesModification of Drosophila p53 by SUMO Modulates Its Transactivation and Pro-Apoptotic FunctionsGustavo FelpeNo ratings yet
- Neuroprotective Effects Of The Pparβ/Δ Antagonist Gsk0660 In In Vitro And In Vivo Parkinson'S Disease ModelsDocument22 pagesNeuroprotective Effects Of The Pparβ/Δ Antagonist Gsk0660 In In Vitro And In Vivo Parkinson'S Disease ModelsAmyNo ratings yet
- Research Paper of Protein Dna InteractionDocument5 pagesResearch Paper of Protein Dna Interactiongvzftaam100% (1)
- DNA ROS p53 2021Document14 pagesDNA ROS p53 2021Maria Elena MaldonadoNo ratings yet
- Ashwagandha 2012 CancerDocument10 pagesAshwagandha 2012 CancercumbredinNo ratings yet
- Nature Targeting Tp53 Cell CycleDocument20 pagesNature Targeting Tp53 Cell CycleKamariah IbrahimNo ratings yet
- Intersectin-2L Regulates Caveola Endocytosis Secondary To Cdc42-Mediated Actin PolymerizationDocument9 pagesIntersectin-2L Regulates Caveola Endocytosis Secondary To Cdc42-Mediated Actin PolymerizationSergeat18BNo ratings yet
- Related Macular Degeneration: A Huge Review and Meta-AnalysisDocument24 pagesRelated Macular Degeneration: A Huge Review and Meta-AnalysisAsad AliNo ratings yet
- CRISPR-CAS9 Telomere RemovalDocument14 pagesCRISPR-CAS9 Telomere RemovalJonathan CorreaNo ratings yet
- LRRK2 Levels in Immune Cells Are Increased in Parkinson's DiseaseDocument12 pagesLRRK2 Levels in Immune Cells Are Increased in Parkinson's DiseaseHợi NguyễnNo ratings yet
- 10 1016@j Urolonc 2019 03 007Document10 pages10 1016@j Urolonc 2019 03 007Marijana KnezovicNo ratings yet
- Identification of Crosstalk Between Phosphoprotein Signaling Pathways in RAW 264.7 Macrophage CellsDocument15 pagesIdentification of Crosstalk Between Phosphoprotein Signaling Pathways in RAW 264.7 Macrophage CellsTauqueer AhmadNo ratings yet
- Gordon Et Al - 2019Document15 pagesGordon Et Al - 2019Chrisjesse NwachukwuNo ratings yet
- Kong Lee Final 1Document10 pagesKong Lee Final 1api-557190611No ratings yet
- Review Article: Immunomodulatory Effects Mediated by DopamineDocument32 pagesReview Article: Immunomodulatory Effects Mediated by DopamineCony GSNo ratings yet
- p53 ThesisDocument4 pagesp53 Thesisjessicahowardknoxville100% (2)
- 8424 FullDocument6 pages8424 FullJulian BarreraNo ratings yet
- Mooth Apn As 2003Document6 pagesMooth Apn As 2003anyasijoNo ratings yet
- 351 FullDocument11 pages351 FullJulia ElenaNo ratings yet
- Biol 230W Lab ReportDocument8 pagesBiol 230W Lab Reportcsh5213No ratings yet
- tmp7C3B TMPDocument9 pagestmp7C3B TMPFrontiersNo ratings yet
- Establishing A Natural History of X-Linked Dystonia ParkinsonismDocument14 pagesEstablishing A Natural History of X-Linked Dystonia ParkinsonismSmilingNo ratings yet
- Of Claudin 1: A Rational Approach of Drug Design For Enteropathogenic E.coli InfectionsDocument9 pagesOf Claudin 1: A Rational Approach of Drug Design For Enteropathogenic E.coli InfectionsmustafaNo ratings yet
- MCB00436-22-Merged - PDF MergedDocument47 pagesMCB00436-22-Merged - PDF MergedHector Ivan Saldivar CeronNo ratings yet
- Ijo 40 6 1889 PDFDocument11 pagesIjo 40 6 1889 PDFshovonNo ratings yet
- Intersectin (ITSN) Family of Scaffolds Function As Molecular Hubs in Protein Interaction NetworksDocument9 pagesIntersectin (ITSN) Family of Scaffolds Function As Molecular Hubs in Protein Interaction NetworksSergeat18BNo ratings yet
- How Do Human Cells React To The Absence of Mitochondrial DNA?Document9 pagesHow Do Human Cells React To The Absence of Mitochondrial DNA?FrontiersNo ratings yet
- Foxp3 ThesisDocument7 pagesFoxp3 Thesisgja8e2sv100% (2)
- Isoform Switching and Exon Skipping Induced by The DNA Methylation Inhibitor 5-Aza-2 - DeoxycytidineDocument9 pagesIsoform Switching and Exon Skipping Induced by The DNA Methylation Inhibitor 5-Aza-2 - Deoxycytidineaam_tabishNo ratings yet
- Biopolym - Cell 2015 31 5suppl 001 enDocument40 pagesBiopolym - Cell 2015 31 5suppl 001 enАнна ШаповаловаNo ratings yet
- Reviews: DNA Microarrays: Translation of The Genome From Laboratory To ClinicDocument8 pagesReviews: DNA Microarrays: Translation of The Genome From Laboratory To ClinicvongoclinhgiangNo ratings yet
- High Expression of Oxidative Phosphorylation Genes Predicts Improved Survival in Squamous Cell Carcinomas of The Head and Neck and LungDocument14 pagesHigh Expression of Oxidative Phosphorylation Genes Predicts Improved Survival in Squamous Cell Carcinomas of The Head and Neck and Lungsalmaa safiraNo ratings yet
- Tissue-Speci Fic Multi-Omics Analysis of Atrial Fibrillation: ArticleDocument15 pagesTissue-Speci Fic Multi-Omics Analysis of Atrial Fibrillation: ArticleAnahí TessaNo ratings yet
- 02 Akita MT 2004Document9 pages02 Akita MT 2004ikramy khalilNo ratings yet
- 016 Scas Abstracts Listed Alphabetically by Abstractspdf 19 2016 Scas AbstractsDocument25 pages016 Scas Abstracts Listed Alphabetically by Abstractspdf 19 2016 Scas AbstractsdssagNo ratings yet
- 1CRISPR To Knowck Down PTENDocument16 pages1CRISPR To Knowck Down PTENLaura AcostaNo ratings yet
- Gene Expression DissertationDocument5 pagesGene Expression DissertationCustomPaperWritersOmaha100% (1)
- PatologíaDocument27 pagesPatologíaZharick Juliana Perdomo FandiñoNo ratings yet
- 2014 GorardiDocument16 pages2014 GorardiAtrocitus RedNo ratings yet
- Mitochondrial Impairment in Microglia Ampli Fies NLRP3 in Ammasome Proin Ammatory Signaling in Cell Culture and Animal Models of Parkinson 'S DiseaseDocument15 pagesMitochondrial Impairment in Microglia Ampli Fies NLRP3 in Ammasome Proin Ammatory Signaling in Cell Culture and Animal Models of Parkinson 'S DiseaseHợi NguyễnNo ratings yet
- Bcri2012 672705Document14 pagesBcri2012 672705Sergeat18BNo ratings yet
- Cryptic Exons - ALS-FTDDocument9 pagesCryptic Exons - ALS-FTDChrisjesse NwachukwuNo ratings yet
- Boumediene Bouzahzah - 2001 - 274 PDFDocument15 pagesBoumediene Bouzahzah - 2001 - 274 PDFarnipahlawaniNo ratings yet
- Genes 13 00165 With CoverDocument12 pagesGenes 13 00165 With CoverMaria Garcia FernandezNo ratings yet
- Evaluation of DNA Damage in COPD Patients and Its Correlation With Polymorphisms in Repair GenesDocument12 pagesEvaluation of DNA Damage in COPD Patients and Its Correlation With Polymorphisms in Repair GenesBambang PrabawigunaNo ratings yet
- TMP FFC1Document28 pagesTMP FFC1FrontiersNo ratings yet
- Sakaeda2002 Article MDR1Up-RegulatedByApoptoticStiDocument7 pagesSakaeda2002 Article MDR1Up-RegulatedByApoptoticStiMarco BrithoNo ratings yet
- Anna U. Bielinska Et Al - Application of Membrane-Based dendrimer/DNA Complexes For Solid Phase Transfection in Vitro and in VivoDocument11 pagesAnna U. Bielinska Et Al - Application of Membrane-Based dendrimer/DNA Complexes For Solid Phase Transfection in Vitro and in VivoHilltopssNo ratings yet
- Leptin 1Document9 pagesLeptin 1vestibuleNo ratings yet
- Luhmann - Ecological CommunicationDocument102 pagesLuhmann - Ecological CommunicationioananeamtNo ratings yet
- Advanced Chemistryprize2013Document12 pagesAdvanced Chemistryprize2013Roberto PinedaNo ratings yet
- For Authors - F1000ResearchDocument5 pagesFor Authors - F1000ResearchRoberto PinedaNo ratings yet
- Rosalind Franklin - The Double Helix and The Wronged Heroine'.Document2 pagesRosalind Franklin - The Double Helix and The Wronged Heroine'.Sergio L.100% (1)
- Formalization of Godel's Ontological ProofDocument2 pagesFormalization of Godel's Ontological ProofYul Enrique Pérez GarcíaNo ratings yet
- Jack 2014 PDFDocument6 pagesJack 2014 PDFRoberto PinedaNo ratings yet
- Me Mory JenDocument17 pagesMe Mory JenRoberto PinedaNo ratings yet
- MamaDocument11 pagesMamaRoberto PinedaNo ratings yet
- Time From Quantum Entanglement: An Experimental IllustrationDocument7 pagesTime From Quantum Entanglement: An Experimental IllustrationRoberto PinedaNo ratings yet
- Aro de VidaDocument4 pagesAro de VidaRoberto PinedaNo ratings yet
- Automated Reasoning in Higher-Order Logic Using The TPTP THF InfrastructureDocument27 pagesAutomated Reasoning in Higher-Order Logic Using The TPTP THF InfrastructureRoberto PinedaNo ratings yet
- Brain Myths: EditorialDocument1 pageBrain Myths: EditorialRoberto PinedaNo ratings yet
- SocNet TheoryAppDocument116 pagesSocNet TheoryAppJosesio Jose Jose Josesio100% (2)
- Menu Restaurante Eye TrackingDocument28 pagesMenu Restaurante Eye TrackingRoberto PinedaNo ratings yet
- Demerger Impact on Shareholder WealthDocument16 pagesDemerger Impact on Shareholder WealthDarshan ShahNo ratings yet
- SRDF S LabDocument9 pagesSRDF S LabUma SekharNo ratings yet
- The CIA Tavistock Institute and The GlobalDocument34 pagesThe CIA Tavistock Institute and The GlobalAnton Crellen100% (4)
- MARGA-Quick Guide enDocument11 pagesMARGA-Quick Guide enKaran TibdewalNo ratings yet
- Chisholm - Referring To Things That No Longer ExistDocument13 pagesChisholm - Referring To Things That No Longer ExistMichele Paolini PaolettiNo ratings yet
- Intro - New Covenant TheologyDocument15 pagesIntro - New Covenant TheologyDavid SalazarNo ratings yet
- Factors Affecting Customer Loyalty to Indosat OoredooDocument13 pagesFactors Affecting Customer Loyalty to Indosat OoredooDede BhubaraNo ratings yet
- Vegetation of PakistanDocument10 pagesVegetation of PakistanAhmad sadiqNo ratings yet
- Sample Thesis Title in Business ManagementDocument6 pagesSample Thesis Title in Business Managementlisabrownomaha100% (2)
- Homemade Water PurifierDocument13 pagesHomemade Water PurifierSherazNo ratings yet
- College Resume TemplateDocument7 pagesCollege Resume Templatevofysyv1z1v3100% (1)
- Professional Teaching ResumeDocument2 pagesProfessional Teaching Resumeapi-535361896No ratings yet
- Definite IntegralsDocument51 pagesDefinite IntegralsKovid BalliNo ratings yet
- Con Law I - Case Cheat SheetDocument22 pagesCon Law I - Case Cheat SheetPriscilla Quansah100% (1)
- Property Management Agreement TemplateDocument2 pagesProperty Management Agreement TemplatemarcelNo ratings yet
- Vodafone - Market Research ProposalDocument4 pagesVodafone - Market Research ProposalNikita Sanghvi100% (2)
- SPE-21639-MSDocument8 pagesSPE-21639-MSehsanNo ratings yet
- Highly Effective College StudentsDocument8 pagesHighly Effective College StudentsPhoenix Kylix0% (1)
- Peta I Think Fizik t4Document18 pagesPeta I Think Fizik t4Yk TayNo ratings yet
- Sale DeedDocument3 pagesSale DeedGaurav Babbar0% (1)
- ĐỀ CHUẨN MINH HỌA SỐ 03Document17 pagesĐỀ CHUẨN MINH HỌA SỐ 03Lê Thị Ngọc ÁnhNo ratings yet
- Khulafa-al-Rashidun, UmarDocument9 pagesKhulafa-al-Rashidun, UmarDuha QureshiNo ratings yet
- Rehotrical AnalysisDocument3 pagesRehotrical AnalysisShahid MumtazNo ratings yet
- Year 9 Autumn 1 2018Document55 pagesYear 9 Autumn 1 2018andyedwards73No ratings yet
- TQM 2 MARKSDocument12 pagesTQM 2 MARKSMARIYAPPANNo ratings yet
- Algebra 2 Function Composition EssayDocument2 pagesAlgebra 2 Function Composition EssayEli PorterNo ratings yet
- CSEC English B June 2013 P2Document7 pagesCSEC English B June 2013 P2Jhanett RobinsonNo ratings yet
- A Practice Teaching Narrative of Experience in Off Campus InternshipDocument84 pagesA Practice Teaching Narrative of Experience in Off Campus InternshipClarenz Jade Magdoboy MonserateNo ratings yet
- Nikita S ResumeDocument2 pagesNikita S ResumeNikita SrivastavaNo ratings yet
- Book Report Template 02Document3 pagesBook Report Template 02JaredNo ratings yet