Professional Documents
Culture Documents
Angio Displa Sia
Uploaded by
BelaFawziaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Angio Displa Sia
Uploaded by
BelaFawziaCopyright:
Available Formats
Background
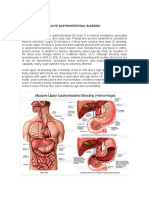
Angiodysplasia is the most common vascular lesion of the gastrointestinal tract, and this condition may be asymptomatic, or it may cause gastrointestinal (GI) bleeding.[1] The vessel walls are thin, with little or no smooth muscle, and the vessels are ectatic and thin (see image below).
Angiodysplasia identified on cecum wall during colonoscopy. Phillips first described a vascular abnormality that caused bleeding from the large bowel in a letter to the London Medical Gazette in 1839. During the 1920s, neoplasms were considered the major source of GI hemorrhage. However, in the 1940s and 1950s, diverticular disease was recognized as an important source of bleeding. In 1951, Smith described active bleeding from a diverticulum visualized through a sigmoidoscope. An association between colonic angiodysplasia and aortic stenosis was described by Heyde in 1958.[2] Vascular abnormalities as a source of active bleeding were controversial. In 1960, Margulis and colleagues identified a vascular malformation in the cecum of a 69-year-old woman who presented with massive bleeding.[3] This diagnosis was accomplished with operative mesenteric arteriography. Galdabini first used the name angiodysplasia in 1974; however, confusion about the exact nature of these lesions resulted in a multitude of terms that included arteriovenous malformation, hemangioma, telangiectasia, and vascular ectasia. These terms have varying pathophysiologies, with a common presentation of GI bleeding. Angiodysplasia is a degenerative lesion of previously healthy blood vessels found most commonly in the cecum and proximal ascending colon. Seventy-seven percent of angiodysplasias are located in the cecum and ascending colon, 15% are located in the jejunum and ileum, and the remainder is distributed throughout the alimentary tract. These lesions typically are nonpalpable and small (< 5 mm). Angiodysplasia is the most common vascular abnormality of the GI tract. After diverticulosis, it is the second leading cause of lower GI bleeding in patients older than 60 years. Angiodysplasia may account for approximately 6% of cases of lower GI bleeding. It may be observed
incidentally at colonoscopy in as many as 0.8% of patients older than 50 years. The prevalence for upper GI lesions is approximately 1-2%. Small bowel angiodysplasia may account for 30-40% of cases of GI bleeding of obscure origin. Retrospective colonoscopic analyses have shown that 12.1% of 642 persons without symptoms of irritable bowel syndrome (IBS), and 11.9% of those with IBS had colonic angiodysplasia.[4] Colonic arteriovenous malformation (AVM) is one of the causes of lower GI bleeding. Unlike small vascular ectasia or angiodysplasia, colonic AVM tends to be solitary, large in size, and identified endoscopically as flat or as an elevated bright red lesion.[5] Angiodysplasia may present as an isolated lesion or as multiple vascular lesions. Unlike congenital or neoplastic vascular lesions of the GI tract, this lesion is not associated with angiomatous lesions of the skin or other viscera. Clinical presentation in patients with angiodysplasia is usually characterized by maroon-colored stool, melena, or hematochezia. Bleeding is usually low grade, but it can be massive in approximately 15% of patients. In 20-25% of bleeding episodes, only tarry stools are passed. Iron deficiency anemia and stools that are intermittently positive for occult blood can be the only manifestations of angiodysplasia in 10-15% of patients. Bleeding stops spontaneously in greater than 90% of cases but is often recurrent.
Pathophysiology
The exact mechanism of development of angiodysplasia is not known, but chronic venous obstruction may play a role.[6, 7] This hypothesis accounts for the high prevalence of these lesions in the right colon and is based on the Laplace law. The Laplace law relates wall tension to luminal size and transmural pressure difference in a cylinder, whereby the wall tension is equal to the pressure difference multiplied by the radius of the cylinder. In the case of the colon, wall tension refers to intramural tension, the pressure difference is that between the bowel lumen and the peritoneal cavity, and cylinder radius is the radius of the right colon. Wall tension is highest in bowel segments with the greatest diameter, such as the right colon. This theory involving chronic venous obstruction suggests that repeated episodes of colonic distention are associated with transient increases in lumen pressure and size., which results in multiple episodes of increasing wall tension with obstruction of submucosal venous outflow, especially where these vessels pierce the smooth muscle layers of the colon. Over many years, this process causes gradual dilation of the submucosal veins and, eventually, dilation of the venules and arteriolar capillary units feeding them. Ultimately, the capillary rings dilate, the precapillary sphincters lose their competency, and a small arteriovenous communication forms. This accounts for the characteristic early-filling vein observed during mesenteric angiography. The developmental theory of angiodysplasia accounts for several clinical and pathologic features, including occurrence in older individuals, location in the cecum and proximal right colon, and prominent submucosal veins that dilate after traversing the muscularis propria. In addition, it also accounts for the lack of pathologic changes in arterioles supplying vascular
ectasias and the absence of any mucosal lesion associated with them. Previous studies demonstrating that colonic motility, increased tension in the bowel wall, and increased intraluminal pressure can diminish venous flow lend further support to this theory. Dilated submucosal veins have been one of the most consistent histologic findings and may represent the earliest abnormality in colonic angiodysplasia. This histologic feature supports the theory of chronic venous obstruction in the genesis of angiodysplasia. Of note, the aforementioned pathophysiologic mechanisms responsible for the development of cecal lesions are unlikely to apply to lesions in the upper GI tract, despite being morphologically identical. A link between a deficiency of high molecular-weight multimers of von Willebrand factor, aortic stenosis, and colonic angiodysplasia has been proposed. Colonic angiodysplasia and true diverticula may be associated.[8] Portal hypertension colopathy, a form of colonic angiodysplasia, has been described.[9] Vascular ectasia of the entire GI tract has been reported in a patient receiving high-dose chemotherapy and autologous stem cell transplantation for relapsing Hodgkin disease.[10]
Epidemiology
Frequency United States
The incidence per 100,000 person-years of hospitalizations due to upper GI ulcer bleeding and perforation has decreased over time, from 54.6 and 3.9 in 1996 (R = 0.944) to 25.8 and 2.9 in 2005 (R = 0.410), respectively. In contrast, the incidence of colonic diverticular and angiodysplasia bleeding per 100 000 person-years increased over time from 3.3 and 0.9 in 1996 (R = 0.443) to 8 and 2.6 in 2005 (R = 0.715), respectively. Recent recorded drug intake showed an increased frequency of anticoagulants with colonic diverticular and angiodysplasia bleeding, whereas NSAID and low-dose aspirin use were more prevalent in peptic ulcer bleeding and colonic diverticular bleeding, respectively.[11] The prevalence of angiodysplasia is 0.8% in healthy patients older than 50 years who are undergoing screening colonoscopy. Foutch et al noted the prevalence of angiodysplasia to be 0.83% from 3 prospective studies in which screening colonoscopies were performed in 964 asymptomatic individuals (mean age, 62 y).[12] Angiodysplasia is the most common reason (50%) for occult GI bleeding. The pooled completion rate was 84%. The pooled retention rates were approximately 2%.[13] Patients with von Willebrand disease may have an increased incidence of GI bleeding from colonic angiodysplasia.[14, 15, 16, 17, 18, 19, 20]
International
No widespread studies to determine the international incidence of angiodysplasia have been conducted, but the incidence probably is similar to that in the United States. Colonic angiodysplasia in Japanese patients is predominantly located in the left colon, whereas in Western patients it is mainly located in the right colon. The percentage of colonic lesions with a size of more than 5 mm or elevated type detected in Japanese patients was significantly higher than in Western patients .[21] Two cases have been reported in Nigeria.[22]
Mortality/Morbidity
Bleeding from angiodysplasia is usually self-limited, but it can be chronic, recurrent, or even acute and life threatening. Approximately 90% of bleeding angiodysplasias spontaneously cease bleeding, presumably because of its venous nature. Mortality is related to the severity of bleeding, hemodynamic instability, age, and the presence of comorbid medical conditions.
Race
No racial predilection exists in cases of angiodysplasia of the colon.
Sex
Angiodysplasia of the colon occurs with equal frequency in men and women.
Age
Most patients found to have angiodysplasia are older than 60 years; of these patients, most are older than 70 years. However, case reports exist of occurrence in young people.[23]
Medscape.com
You might also like
- 58 Lower Gastrointestinal BleedDocument4 pages58 Lower Gastrointestinal BleedLuphly TaluvtaNo ratings yet
- Orem's Self-Care Deficit Nursing TheoryDocument30 pagesOrem's Self-Care Deficit Nursing TheoryDairyl Tagaro100% (1)
- Epilepsy and Seizure DisordersDocument38 pagesEpilepsy and Seizure DisordersMalueth AnguiNo ratings yet
- Health & Safety Policy QEB Standards 13Document2 pagesHealth & Safety Policy QEB Standards 13QBE European Operations Risk ManagementNo ratings yet
- Project ProposalDocument4 pagesProject ProposalLeonard Andrew Manuevo100% (1)
- Boli Digestive Functionale 2018Document90 pagesBoli Digestive Functionale 2018Cabel TeodorNo ratings yet
- Heyde Syndrome - A Common Diagnosis in Older Patients With Severe Aortic StenosisDocument4 pagesHeyde Syndrome - A Common Diagnosis in Older Patients With Severe Aortic StenosisAbdi KebedeNo ratings yet
- Colonic Ischemia - UpToDateDocument35 pagesColonic Ischemia - UpToDateWitor BelchiorNo ratings yet
- Lower Gastrointestinal BleedingDocument18 pagesLower Gastrointestinal Bleedingosama nawaysehNo ratings yet
- Uppergastrointestinalbleeding: Marcie Feinman,, Elliott R. HautDocument11 pagesUppergastrointestinalbleeding: Marcie Feinman,, Elliott R. HautjoseNo ratings yet
- Varices: Esophageal, Gastric, and RectalDocument18 pagesVarices: Esophageal, Gastric, and RectalHưng Nguyễn KiềuNo ratings yet
- Portal HypertensionDocument13 pagesPortal HypertensionCiprian BoesanNo ratings yet
- An Unusual Case of Polycythemia Vera With A Complication of Pancreatic PseudocystDocument3 pagesAn Unusual Case of Polycythemia Vera With A Complication of Pancreatic PseudocystAgus PrimaNo ratings yet
- Mallory Weiss Syndrome - StatPearls - NCBI BookshelfDocument9 pagesMallory Weiss Syndrome - StatPearls - NCBI BookshelfDaniela EstradaNo ratings yet
- Acute GI BleedingDocument13 pagesAcute GI BleedingDavid RyanNo ratings yet
- 2005 Rolebleeding PDFDocument5 pages2005 Rolebleeding PDFhaninamaulianiNo ratings yet
- Left SidedDocument3 pagesLeft SidedElisabeth ZzMick GtNo ratings yet
- Endoscopicmanagementof Portalhypertension-Related Bleeding: Andrew Nett,, Kenneth F. BinmoellerDocument17 pagesEndoscopicmanagementof Portalhypertension-Related Bleeding: Andrew Nett,, Kenneth F. BinmoellerAlonso CayaniNo ratings yet
- Me DCL Inn Avarice Al BleedingDocument24 pagesMe DCL Inn Avarice Al BleedingKarina WibowoNo ratings yet
- Cecum Dieulafoy's Lesion Presenting With Recurrent Lower Gastrointestinal Bleeding: Case ReportDocument5 pagesCecum Dieulafoy's Lesion Presenting With Recurrent Lower Gastrointestinal Bleeding: Case ReportSabrina JonesNo ratings yet
- Lower GIT BleedingDocument89 pagesLower GIT Bleedingمجاهد إسماعيل حسن حسينNo ratings yet
- Management of Patient With Gastrointestinal BleedingDocument14 pagesManagement of Patient With Gastrointestinal BleedingPulkit MandalNo ratings yet
- Abdominal ImagingDocument6 pagesAbdominal Imagingmihaela2786No ratings yet
- Diverticular Disease of The ColonDocument9 pagesDiverticular Disease of The ColondrheayNo ratings yet
- Upper Gastrointestinal Bleeding (UGIB) : Practice EssentialsDocument52 pagesUpper Gastrointestinal Bleeding (UGIB) : Practice EssentialsrishaNo ratings yet
- Acute Gastrointestinal Bleeding Upper GI BleedingDocument18 pagesAcute Gastrointestinal Bleeding Upper GI BleedingAldwin AyuyangNo ratings yet
- Ascitis ImportanteDocument17 pagesAscitis Importantedanyescalerapumas24No ratings yet
- Esophageal Varices: Pathophysiology, Approach, and Clinical DilemmasDocument2 pagesEsophageal Varices: Pathophysiology, Approach, and Clinical Dilemmaskaychi zNo ratings yet
- Vascular Disease of The BowelDocument28 pagesVascular Disease of The BowelOlga GoryachevaNo ratings yet
- Upper Gastrointestinal Bleeding: EL IlcoxDocument1 pageUpper Gastrointestinal Bleeding: EL IlcoxdaisyNo ratings yet
- Aortoiliac Occlusive DiseaseDocument37 pagesAortoiliac Occlusive Diseasewolff_512No ratings yet
- Left-Sided Portal Hypertension: A Clinical Challenge: Hipertensão Portal Esquerda: Um Desafio ClínicoDocument3 pagesLeft-Sided Portal Hypertension: A Clinical Challenge: Hipertensão Portal Esquerda: Um Desafio ClínicomichaelqurtisNo ratings yet
- VASCULITIS by Dr. AJ. 1Document34 pagesVASCULITIS by Dr. AJ. 1Abira KhanNo ratings yet
- AscitesascitesDocument15 pagesAscitesascitesKevinJuliusTanadyNo ratings yet
- Upper GI Bleeding Causes, Signs, Risk Factors & ManagementDocument46 pagesUpper GI Bleeding Causes, Signs, Risk Factors & ManagementRashed ShatnawiNo ratings yet
- GIB ReportDocument2 pagesGIB ReportRani Gayle RiveraNo ratings yet
- Modified Alvarado Score and Its Application in The Diagnosis of Acute AppendicitisDocument2 pagesModified Alvarado Score and Its Application in The Diagnosis of Acute AppendicitisanunadNo ratings yet
- Pathophysiology of Variceal Bleeding in CirrhoticsDocument8 pagesPathophysiology of Variceal Bleeding in Cirrhoticsanon_914506742No ratings yet
- Causes of Upper Gastrointestinal Bleeding in Adults UpToDateDocument37 pagesCauses of Upper Gastrointestinal Bleeding in Adults UpToDateJodene Rose RojasNo ratings yet
- Anasarca - StatPearls - NCBI BookshelfDocument5 pagesAnasarca - StatPearls - NCBI Bookshelfemail lombaNo ratings yet
- Vasculer Anomly of IntestineDocument38 pagesVasculer Anomly of Intestineعبد الله مازن موسى عبدNo ratings yet
- Colitis Isquemica PDFDocument7 pagesColitis Isquemica PDFWaldo ReyesNo ratings yet
- Esophageal VaricesDocument4 pagesEsophageal Varicesbrian3442No ratings yet
- Robertson 2017Document10 pagesRobertson 2017tnsourceNo ratings yet
- Upper Gastrointestinal Bleeding.Document13 pagesUpper Gastrointestinal Bleeding.Gieselle Scott100% (1)
- Acute GIDocument8 pagesAcute GIFerry SahraNo ratings yet
- Chapter 2 Malignant Pleural EffusionDocument14 pagesChapter 2 Malignant Pleural EffusionDaniel EnglishNo ratings yet
- Webb 2641Document12 pagesWebb 2641owcordal7297No ratings yet
- Abdominal ImagingDocument16 pagesAbdominal Imagingfrancu60No ratings yet
- PMR and TADocument11 pagesPMR and TAハルァン ファ烏山No ratings yet
- Upper Gi Bleed: History, Pe, Pathophysiology, Diagnostics and TreatmentDocument6 pagesUpper Gi Bleed: History, Pe, Pathophysiology, Diagnostics and TreatmentJennicaNo ratings yet
- 4.1 Bicuspid Aortic ValveDocument33 pages4.1 Bicuspid Aortic ValveAbnet WondimuNo ratings yet
- Diverticulosis and Diverticulitis-1Document11 pagesDiverticulosis and Diverticulitis-1mgoez077No ratings yet
- Tugas Gadar EndokrinDocument10 pagesTugas Gadar Endokrinokie oktaviaNo ratings yet
- Ulceras Vasculares en MiDocument11 pagesUlceras Vasculares en MiKarilNo ratings yet
- Jaundice: Key Teaching PointsDocument12 pagesJaundice: Key Teaching PointsSebastian Cebrian GuerreroNo ratings yet
- Esophageal and Gastric VaricesDocument15 pagesEsophageal and Gastric VaricesDumbo D' CatNo ratings yet
- Case Alcohol Abuse and Unusual Abdominal Pain in A 49-Year-OldDocument7 pagesCase Alcohol Abuse and Unusual Abdominal Pain in A 49-Year-OldPutri AmeliaNo ratings yet
- Primary Aortoenteric Fistula Due to Septic Aortitis CaseDocument5 pagesPrimary Aortoenteric Fistula Due to Septic Aortitis CaseKintrili AthinaNo ratings yet
- Chylous, Bloody, and Pancreatic Ascites - UpToDateDocument26 pagesChylous, Bloody, and Pancreatic Ascites - UpToDateAndrea RodríguezNo ratings yet
- Colonoscopy findings in young patients with rectal bleedingDocument6 pagesColonoscopy findings in young patients with rectal bleedingpratiwifatmasariNo ratings yet
- Key factors in evaluating short statureDocument8 pagesKey factors in evaluating short statureBelaFawziaNo ratings yet
- Neuroendocrine Regulation of The Stress ResponseDocument5 pagesNeuroendocrine Regulation of The Stress ResponseBelaFawziaNo ratings yet
- Key factors in evaluating short statureDocument8 pagesKey factors in evaluating short statureBelaFawziaNo ratings yet
- ConstipationDocument7 pagesConstipationBelaFawziaNo ratings yet
- Angio Displa SiaDocument4 pagesAngio Displa SiaBelaFawziaNo ratings yet
- Angio Displa SiaDocument4 pagesAngio Displa SiaBelaFawziaNo ratings yet
- Oxford Textbook of Old Age PsychiatryDocument7 pagesOxford Textbook of Old Age PsychiatryCengizhan ErNo ratings yet
- Exam ChecklistDocument13 pagesExam Checklistmahmoud selimNo ratings yet
- Psych 1Document7 pagesPsych 1Michael Baylon DueñasNo ratings yet
- PPE Use, Disposal & Recycling SurveyDocument2 pagesPPE Use, Disposal & Recycling SurveySamantha DomileNo ratings yet
- Pengetahuan Ergonomi Dan Postur Kerja Perawat Pada Perawatan Luka Dengan Gangguan Muskuloskeletal Di Dr. H. Koesnadi BondowosoDocument5 pagesPengetahuan Ergonomi Dan Postur Kerja Perawat Pada Perawatan Luka Dengan Gangguan Muskuloskeletal Di Dr. H. Koesnadi BondowosoImanuel SihotangNo ratings yet
- Electro-Chemiluminescence Immunoassay (ECLIA) For The Quantitative Determination of CA 15-3 in Human Serum and PlasmaDocument2 pagesElectro-Chemiluminescence Immunoassay (ECLIA) For The Quantitative Determination of CA 15-3 in Human Serum and PlasmayantuNo ratings yet
- Giant Ovarian Cyst Mimicking AscitesDocument3 pagesGiant Ovarian Cyst Mimicking AscitesbellastracyNo ratings yet
- DORC HSE Department Contractors' Safety MeetingDocument63 pagesDORC HSE Department Contractors' Safety MeetingJosiahNo ratings yet
- Research in Social and Administrative PharmacyDocument4 pagesResearch in Social and Administrative PharmacyAndrea MendozaNo ratings yet
- Leadership Accountability Nurs631Document6 pagesLeadership Accountability Nurs631api-241398334No ratings yet
- McIlwaine Et Al-2015-Cochrane Database of Systematic ReviewsDocument74 pagesMcIlwaine Et Al-2015-Cochrane Database of Systematic Reviewsangelica barrazaNo ratings yet
- Aorn Laser SafetyDocument16 pagesAorn Laser SafetyAmanda RapaNo ratings yet
- WB CE Act Registration RegulationDocument42 pagesWB CE Act Registration RegulationDipak Ranjan MukherjeeNo ratings yet
- Including Disabled People in Sanitation and Hygiene ServicesDocument8 pagesIncluding Disabled People in Sanitation and Hygiene ServicesRae ManarNo ratings yet
- Frequency of Over Eruption in Unopposed Posterior TeethDocument7 pagesFrequency of Over Eruption in Unopposed Posterior TeethDaniel Lévano AlzamoraNo ratings yet
- An Auricular Marker For Covid-19: Nadia Volf, MD, PHD, Valery Salques, MD, and Anne Lassaux, MDDocument2 pagesAn Auricular Marker For Covid-19: Nadia Volf, MD, PHD, Valery Salques, MD, and Anne Lassaux, MDyan92120No ratings yet
- Managing The Health Insurance PortfolioDocument3 pagesManaging The Health Insurance PortfolioShantanuNo ratings yet
- Causes of an Opacified HemithoraxDocument17 pagesCauses of an Opacified HemithoraxKevin SurjadiNo ratings yet
- PhysAssessAbdomen 1Document21 pagesPhysAssessAbdomen 1David DvoskineNo ratings yet
- Karya Ilmiah Akhir NersDocument27 pagesKarya Ilmiah Akhir NersSyaputri ChairulNo ratings yet
- NEBOSH IGC1 Past Exam Paper March 2012Document3 pagesNEBOSH IGC1 Past Exam Paper March 2012Seleni100% (1)
- Talkshow #5Document11 pagesTalkshow #540Thiên TrangB2No ratings yet
- How To Maintain Interpersonal RelationshipsDocument5 pagesHow To Maintain Interpersonal RelationshipsRichard BryanNo ratings yet
- Albert SchweitzerDocument2 pagesAlbert SchweitzerStar KingsNo ratings yet
- Professional Adjustment in NursingDocument47 pagesProfessional Adjustment in NursingAnning HagarNo ratings yet