Professional Documents
Culture Documents
Common Genetic Disease Related Hormonal and Metabolism: DR Erna Mirani, M.Si - Med
Uploaded by
Fahmi Nur Zaman0 ratings0% found this document useful (0 votes)
25 views63 pageskuliah pakar
Original Title
Kuliah Genetik Erna
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentkuliah pakar
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
25 views63 pagesCommon Genetic Disease Related Hormonal and Metabolism: DR Erna Mirani, M.Si - Med
Uploaded by
Fahmi Nur Zamankuliah pakar
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 63
Common genetic disease related
hormonal and metabolism
Dr Erna Mirani, M.Si.Med
Tujuan Pembelajaran
Mengetahui kelainan kromosomal yang
menyebabkan kelainan kongenital
Mengetahui kelainan metabolisme dan
hormonal yang berasal dari kelainan genetik
WHAT CAUSES GENETIC CONDITIONS?
Heritable conditions
Due to a mutation in a single gene
Chromosomal conditions
Packaging errors caused by structural changes in the chromosomes or
the gain or loss of whole chromosomes, (or parts of chromosomes)
during either the formation of the egg or sperm or at conception
Multifactorial conditions
Due to the interaction of the genetic information and environmental
factors such as diet, chemical exposure and lifestyle
WHAT CAN BE DONE ABOUT GENETIC CONDITIONS?
(a) Prevention
to prevent about 70% of the cases of spina bifida in babies if women take the vitamin
folic acid before, and continue it during early pregnancy
(b) Early Diagnosis and Treatment screened for
phenylketonuria (PKU). Diagnosis and treatment within the first month of life are crucial
to avoid intellectual disability.
(c) Genetic Counselling
to families and individuals that have concerns about a condition in their family which
may have a genetic basis.
to provide information and supportive counselling so that families may be better able to
understand, and adjust to, the diagnosis of a genetic condition
Genetic testing, can also be organised on the basis of informed consent These tests can
be used prior to conception, to determine a couple's risk of having an affected child;
during pregnancy, to identify possible genetic disorders; and at birth or later in life, to
assess an individual's probability of developing a disorder.
(d) Support Groups
Single-gene (also called Mendelian or monogenic) inherited in recognizable
patterns: autosomal dominant, autosomal recessive, and X-linked.
Multifactorial (also called complex or polygenic is caused by a combination
of environmental factors and mutations in multiple genes. For example,
different genes that influence breast cancer susceptibility have been
found on chromosomes 6, 11, 13, 14, 15, 17, and 22.
Mitochondrial : Leber optic atrophy , Mitochondrial myopathies ,Pearson
syndrome
Chromosomal :
Abnormalities in chromosome structure as missing
extra copies
gross breaks
rejoinings (translocations), can result in disease. Down syndrome or
trisomy 21 is a common disorder
Chromosomal disorders
The two most common :
aneuploidies, trisomies and extra sex chromosomes, can be due to
maternal or paternal factors, including advanced age.
A number of aneuploidies can be attributed to dispermywhere two
sperm fertilized one egg. The resulting genetic disorders can occur due
to a spontaneous mutation, and a familial tendency towards these
disorders cannot always be found.
Aneuploidy of the sex chromosomes can cause abnormal genital
development, sterility, and other growth problems. The most common
such aberration are multiple X syndromes. ;
Triple X females can bear normal children.
Males with an XXY aneuploidy are afflicted with Klinefelter's syndrome,
have small testes and cannot produce sperm.
Men with XYY aneuploidy are born more frequently (about 1 in every 200-
1,000 males) than most aneuploidies, and controversy exists as to
whether these individuals have a higher criminal tendency than the rest
of the male population.
Trisomi
Trisomies make up to 52% of chromosomal abnormalities, with
trisomies 14, 15, 16, 18, 21, and 22 being the most frequent.
Live-born children with autosomal aneuploidies have trisomy 13, 18,
or 21, and all have some mental retardation.
Trisomy 13 (Patau's syndrome) is characterized by retarded growth,
cleft lip, small head and chin, and often polydactyly.
Trisomy 18 (Edward's syndrome) is marked by severe, variable
abnormalities of the head, thumbs, ears, mouth, and feet.
Trisomy 21 (Down syndrome) occurs equally in all ethnic groups, and
is closely related to increased maternal age. Children with Down
syndrome can have poor muscle tone, a flattened face, extra folds of
skin at the eyes, low-set ears, visible (Brushfield) spots on the iris of
their eyes, and a single crease along the palm of their hands.
Kelainan autosomal
Gambaran dismorfik jelas
Mengikuti pola pertumbuhan khas
TB dewasa L = 155 cm P = 145 cm
Penyebab perawakan pendek tak jelas
Tx/ dipertimbangkan hormon pertumbuhan
Sindrom Down
Trisomy 21
The most frequent viable chromosome disease.
Like other inborn autosomal chromosome diseases,
associates dysmorphia + psycho-motor delay, and
possible visceral malformations (found in more than
1/3 of cases)
a medico-pedagogic care and follow up must be
undertaken.
Trisomy 21
1,5 /1 000 births.
Sex ratio: 3 males/2 females.
Increased median maternal age (34 years).
Maximal trisomy 21 births from mothers aged:
The risk increases with maternal age:
<0.1% below 30 yrs;
between 0.1% and 1% at ages 30-40
0.2% at 34 yrs,
0.5% at 38 yrs,
0.7% at 39 yrs);
>1% above 40 yrs
5% at 46 yrs,
15% at 50 yrs.
CLINICAL EXAMINATION
1 - Dysmorphic syndrome
frequent microcephaly, short neck, flat occiput and brachycephaly;
moon-shaped face;
flat nasal bridge;
"socket" nostrils;
hypertelorism (or pseudo-hypertelorism);
epicanthus (regresse with age);
upward slanting palpebral fissures;
Brushfield spots in the iris (pathognomonic, detectable in blue eyes).
macroglossia; glossitis exfoliativa (geographic tongue); scrotal tongue at late
childhood and in adulthood;
mouth frequently open; frequently open mouth;
narrow/ high arched palate; high arched narrow palate;
late appearing/malformed teeth (numerical anomalies, agenesis of lateral
incisors...);
hands and feet:
short and broad;
brachymesophalangia of the 2nd and 5th fingers;
clinodactyly of the 5th finger;
flat feet;
first toe set apart from the others by a gap, with a crease.
dry skin, mottled skin (livedo), with frequent infections around orifices.
hyperlaxity of ligaments.
frequent umbilical hernia.
2 - Psycho-motor delay (constant):
hypotonia +++ at birth (hold his head at 6 mths, sits at age 1
yr, walks at age 2 yrs).
the mental retardation, not obvious in the infant, will soon
become manifest.
children's behaviour:
affectionate, gentle, cheerful;
language difficulties;
like to play, to mime, to tidy up meticulously;
normal memory.
seizures (in 3% to 9%, as compared to 1% in the general
population).
- Malformations (45% of cases):
Heart (40%):
atrioventricular septal defect (10 %);
ventricular septal defect (10 %);
patent foramen ovale (5 %);
persistence of ductus arteriosus (5 %)...
Digestive (10 %):
duodenal stenosis (1/3 of duodenal stenosis are found in trisomy
21patients);
imperforate anus...
Ocular:
cataract (congenital or acquired);
astigmatism;
myopia;
strabismus;
congenital glaucoma;
nystagmus.
- Malformations (45% of cases):
Hematologic:
transient leukemoid reaction may occur
sometimes with a relapse as acute leukemia
(lymphoblastic (ALL) or more frequently non-
lymphoblastic (ANLL) leukemias; M7-ANLL
(megakaryocytic) is particularly frequent. Watch the
hypersensitivity to methotrexate.
Immunological:
tuberculine hyporeactivity;
immune deficiency.
Metabolic:
hyperuricemia;
abnormal glycemia;
increased TSH (frequent); hypo or hyper thyroidy.
Free and homogeneous trisomy 21
(92,5 % of cases):
sporadic (de novo) cases.
role of maternal age (see above in epidemiology).
recurrence risk: 1 to 2 %.
karyotype: 47,XY,+21 ou 47,XX,+21.
due to meiotic non-disjunction:
of maternal origin:
- lst division: 70 %
- 2nd division: 20 %
of paternal origin:
- lst division: 5 %
- 2nd division: 5 %
Free trisomy 21 in mosaic (2,5 % of
cases):
sporadic cases.
karyotype: 46, XY / 47, XY,+21 or 46, XX / 47,
XX,+21.
post zygotic event (mitotic).
most often, the phenotype is typical, at times
attenuated.
Trisomy 21 due to translocation
de novo or transmitted from a parental translocation (being a balanced
translocation in the parent); genetic coonseling is especially needed in the latter
case.
karyotype with 46 chromosomes; the extra chromosome 21 is most often
translocated with another acrocentric (groupe D: 14, 13 or 15 or groupe G: 21 or
22) chromosome; example: 46, XY, t(14;21).
genetic counseling and recurrence risk:
t(Dq;21q) et t(21q;22q)
of maternal origin: risk = 15 %
of paternal origin: risk = 5%
t(21q;21q): risk = 100 %:
either --> trisomy 21
or --> spontaneous miscarriage (monosomy 21).
Other:
partial trisomy 21 (rare). -->(the segment responsible for most of the
syndrome/phenotype is band 21q22.3.
associated with other chromosome anomalies (rare).
EVOLUTION
statural delay (adult = 1,50 m); weight excess (--> diet).
voice becomes hoarse.
puberty is delayed but normal; poor libido;
fecondity in the female ( --> contraception).
hypothyroidy, Basedow (--> T3, T4, TSH, reverse T3 regular determination).
mental development:
(IQ) = 50 (mean (and median)); between 30 and 80; vary according to age);
social insertion: partly according to the familial environment, the guidance
and reassurance that the family receives, and according to the medical,
paramedical, and pedagogic cares
psychomotor therapy from the age of 6 mths, early aging:
behaviour may suddenly switch from that of a happy and sociable child to a
sad, inactive and inexpressive adult;
risk of senile dementia (Alzheimer disease).
PROGNOSIS
life expectancy, formerly poor, has greatly increased,
due to antibiotherapy and surgery.
prognosis can be impaired by:
1 - the extreme susceptibility to infections.
2 - malformations, cardiac malformations in
particular.
3 - acute leukemia (in 1 % of trisomy 21
infants/children, i.e. 20 times more frequently than
in the general population).
TRISOMY 13 (Patau syndrome)
I. Epidemiology:
0.1 / 1 000 births.
increased parental age.
normal pregnancy duration.
life expectancy: frequently found in early miscarriages, and in late
miscarriages; stillbirths are common, and babies often die in the neonatal
period; very few reach adulthood.
II. Clinics:
microcephaly, receding forehead.
microphtalmia/anophtalmia, colobomata of the iris, cataract.
arrhinencephaly, probocis.
hypotelorism.
scalp defect (in relation with neural tube fusion defects).
hare-lip / cleft palate.
umbilical hernia: 1/3 of cases.
genitalia: cryptorchidy in the male, uterus bicornis (constant) and vagina
duplex (often) in the female.
fingers in flexion position; postaxial polydactyly 80 % (hands and feet); club
foot; dermatoglyphics: axial triradius in t"; thenar pattern.
III. Malformations: constant, heavy, leading to early death in most of the
cases.
Central nervous system : arhinencephaly (50 %),hypoplasia of the corpus
callosum (20 %).hypoplasia of the frontal lobe.spina bifida.
Ocular: micro/anophtalmia (90 %). coloboma. retinal dysplasia. luxation
or absence of lens.
Cardiac (constant): ventricular septal defect. patent foramen ovale.
persistence of ductus arteriosus tetralogy of Fallot.
Renal (50 %): hydronephrosis. polykystic kidneys ...
Digestive (50 %): malrotation of the intestine. malformation of the
pancreas. gallbladder agenesis.
Bones: spina bifida. rib malformations.
IV. Karyotype:
most often free and homogenous trisomy.
sometimes translocation t(13q 14q).
sometimes mosaic trisomy.
- TRISOMY 18
Epidemiology:
0.2 / 1 000 births.
increased parental age.
pregnancy duration is often prolonged.
life expectancy: frequently found in miscarriages; stillbirths are
common, and babies often die in the neonatal period; very few reach
adulthood.
II. Clinics:
hydramnios; single umbilical artery frequently.
low birth weight: 2,3 kg.
constant sign: hypoplasia of the first branchial arch, which implicates:
--> low set ears
- TRISOMY 18
short thorax and sternum, making the abdomen
looking long.
hernias: diaphragmatic, umbilical, inguinal.
cryptorchidism (30 %).
clubfoot; irreducible flexion of forearms;
dysplastic nails, absence of distal flexion
crease of fingers; clenched fingers with
overlap of the 2nd and 5th onto the 3rd and
4th; dermatoglyphics: frequency of arches
- TRISOMY 18
Malformations: constant, heavy, leading to early death in
most of the cases.
Cardiac: constant.
ventricular septal defect.
patent foramen ovale.
persistence of ductus arteriosus
valves anomaly, in particular mitral valve
Renal (1/3): mostly horseshoe kidney, hydronephrosis, polykystic
kidneys, hyploplastic kidneys.
Digestive: frequent; Meckel, anal atresia; pancreas anomalies.
Brain
Bones: spina bifida, hemivertebrae , absence of clavicle.
IV. Karyotype:
most often free and homogenous trisomy.
frequency of doubles aneuploidies and mosaics.
Sindrom Turner
Kromosom 45 X atau mosaik
Patogenesis tak jelas
Mungkin kelainan fungsional
poros hipotalamus hipofisis
Respon terhadap GHRH akut rendah
Tx/
Hormon pertumbuhan
steroid anabolik
Estrogen dosis rendah
Aneuploidy
diagnosis
The diagnosis can be evoked either:
in the newborn (from dysmorphia and/or malformations), or:
in the girl (from growth retardation, impuberism).
neo-natal form:
prenatal (and postnatal) growth retardation
single umbilical artery frequently.
Bonnevie-Ullrich (BU) status associating:
lymphoedema of hands and feet (tough, non inflammatory, regressive at
age 2 yrs).
excess of skin and webbed skin on the nucha (pterygium colli).
1/3 of BU are found in Turner syndrome, and 75 % of Turner have a BU In
the presence of this symptomatology, a karyotype will be undertaken and
(cardiac, renal) malformations will be searched for.
Malformations:
cardiovascular (20-30%): aortic coarctation (10-15 %) which may lead
to death by dissection or rupture of the aorta; bicuspid aortic valve;
left superior vena cava, and other malformations; in the presence of
aortic coarctation in a girl, a Turner syndrome must be evoked.
renal (40-50 %): horseshoe kidney, hydronephrosis...
congenitally dislocated hip, scoliosis
sense-organs: deafness (impaired hearing in up to 40%), myopia,
cataract, strabismus.
X linked recessive inherited traits have the same frequency in Turner
syndrome and in the male, since they both have only 1 X; this
frequency is that of the allele (e.g. daltonism, hemophilia, Duchenne
de Boulogne myopathy...).
karyotype:
45, X homogeneous: 55 % of cases.
isochromosomes: i(Xp), i(Xq); deleted
chromosomes: del (Xp), del (Xq); rings: r(x);
mosaicisms... --> phenotypes are more or less
evocative of Turner syndrome some patients
having been fertile.
most of the phenotypic traits are due to Xp
deletion, and only ovarian failure is consistently
associated with Xq deletions.
Assessments:
ovarian failure (sex steroid deficiency and amenorrhea).
streak gonads (germinal cells regress at the 3rd month in utero; biopsy
is not needed).
impaired glucose tolerance; hypertension (20-30%).
autoimmune thyroid disease (T4, TSH, thyroid-antibody titer
determinations).
X-rays (skeleton, urinary system, heart).
Differential diagnosis:
other disorders with Bonnevie-Ullrich status.
gonadic dysgenesia.
other disorders with primary amenorrhea; e. g.: XY females (sex
reversal).
Grafik pertumbuhan linier
Sindrom Turner
Tinggi akhir 130 - 148 cm
Grafik pertumbuhan normal
Sindrom Klinefelter (47 XXY )
Hanya pada anak laki-laki.
Retardasi mental (tidak semua)
Tinggi dengan proporsi eunuchoid
Ginekomastia
Testes kecil dan keras volumenya tidak lebih
dari 6 ml.
Klinefelter syndrome is a syndrome of a
normal or gynecoid male with normal
intelligence or mild retardation, infertility, and
possible behaviour or psychiatric problems,
due to a chromosome imbalance: 47, XXY and
variants.
Epidemiology:
1.5 /1 000 male births. ,increased maternal age. ,the extra X comes more often
from the mother.
Clinical ascertainment/examination:
wide variability in clinical expression, rarely diagnosed in childhood (from
mental retardation or non specific anomalies of genitalia),
more often at puberty (from gynecomastia, small testes),
or when consulting for infertility.
physical aspect is often normal,
they may present with tallness and macroskelia,
or with gynecoidy (gynecoid obesity: 25 %; gynecomastia: 15-25 %; bi-
trochanteric diameter > bi-acromial diameter).
normal penis. small, indolent testes. normal or rare, feminine shaped pubic
pilosity.
libido diminished; impotence at age 30 yrs is frequent.
sterility. normal or moderately delayed intellectual development.
dyslexia/dysphasia and frontal-executive dysfunction. psychiatric
behaviour is not rare.
50-fold increased risk of developing breast cancer as compared to normal
males (and 8 times less than in females, as the womens risk is 400 times that
of men) (nearly 10% of breast cancers in males are found in Klinelter patients).
Diagnosis: the karyotype:
47 XXY homogeneous: 80 % of cases.
XXXY, XXXXY, XXYY: 10 %.
in mosaic: 5-10 % (may (rarely) be fertile).
Assessments:
high gonadotropins and low testosterone plasma levels.
azoospermia in most non-mosaic cases; however,
intratesticular residual foci of spermatogenesis may
occasionally be found, and mature spermatozoa may
permit paternity using intracytoplasmic sperm injection.
biopsy (not needed): seminiferous tubes atrophia, Leydig
hyperplasia .
Treatment: testosterone replacement therapy to correct the
androgen deficiency and to provide virilization; can also has
positive effects on mood and self-esteem.
Metabolic disease
What is a metabolic disease?
Garrods hypothesis
product deficiency
substrate excess
toxic metabolite
A
D
B
C
Inborn errors of metabolism
Definition:
Inborn errors of metabolism occur from a group of
rare genetic disorders in which the body cannot metabolize
food components normally. These disorders are usually
caused by defects in the enzymes involved in the
biochemical pathways that break down food components.
Alternative Names:
Galactosemia - nutritional considerations; Fructose
intolerance - nutritional considerations; Maple sugar urine
disease (MSUD) - nutritional considerations;
Phenylketonuria (PKU) - nutritional considerations;
Branched chain ketoaciduria - nutritional considerations
Background:
Inborn errors of metabolism (IEMs) individually are
rare but collectively are common. Presentation can occur at
any time, even in adulthood.
Diagnosis does not require extensive knowledge of
biochemical pathways or individual metabolic diseases.
An understanding of the broad clinical manifestations
of IEMs provides the basis for knowing when to consider
the diagnosis.
Most important in making the diagnosis is a high
index of suspicion.
Successful emergency treatment depends on prompt
institution of therapy aimed at metabolic stabilization.
A genetically determined
biochemical disorder in which a
specific enzyme defect produces a
metabolic block that may have
pathologic consequences at birth
(e.g., phenylketonuria) or in later life
(e.g., diabetes mellitus); called also
enzymopathy and genetotrophic
disease.
Classification
Inborn Errors of Small molecule Metabolism
Example: Galactosemia
Lysosomal storage diseases
Example: Gaucher's Disease
Disorders of Energy Metabolism
Example Glycogen Storage Disease
Other more rare classes of metabolism error
Paroxysmal disorders
Transport disorders
Defects in purine and pyrimidine metabolism
Receptor Defects
PKU
Pathophysiology:
Single gene defects result in abnormalities in the
synthesis or catabolism of proteins, carbohydrates, or fats.
Most are due to a defect in an enzyme or transport
protein, which results in a block in a metabolic pathway.
Effects are due to toxic accumulations of substrates
before the block, intermediates from alternative metabolic
pathways, and/or defects in energy production and utilization
caused by a deficiency of products beyond the block.
Nearly every metabolic disease has several forms that
vary in age of onset, clinical severity and, often, mode of
inheritance.
Metabolic disease (AR)
Galactosemia :
- cannot metabolize galactose, the sugar found in milk
- mental retardation may result if normal milk is not avoided by people
with this rare disease
PKU :
cannot convert phenylalanine to tyrosine.
The build-up of phenylalanine leads to severe mental retard.
1 in 50 Caucasians.
can be controlled by diet/ A phenylalanine-free diet containing
sufficient amino acids is available for people diagnosed with PKU.
Since 1961, a test has been available to readily screen newborns for
PKU from a blood test, and most states perform this test routinely.
Syndrom related hormonal
disorders
Sindrom Johanson-Blizzard
retardasi mental derajad bervariasi
alae nasi hipoplastik atau aplstik
gigi susu hipoplastik, gigi permanen (-)
kriptorkidisme
mikropenis
vagina rangkap atau septum
hidronefrosis
hipotiroidisme primer
insufisiensi pankreas
Marfan's syndrome
(arachnodactyly)
long, thin arms, legs, and fingers.
stoop-shouldered and have blue sclera of the eyes.
a high incidence of eye and aortic heart problems.
Statistics show some correlation between older
fathers and offspring with Marfan's.
Not all people with Marfan's inherit it from a parent
about 15% of Marfan's cases are caused by a fresh
mutation in the same locus.
Pathogenesis
The fibrillin-1 (FBN1) gene
encodes the glycoprotein
fibrillin, a major building
block of microfibrils
The microfibrils constitute
the structural components
of the suspensory ligaments
of the lens, and serve as a
substrates for elastin in the
aorta and the other
connective tissues
Cystic Fibrosis
CF is one of the most common autosomal recessive
diseases in Caucasian children
in the U.S. About 4-5% of Caucasians carry this
recessive gene on chromosome 7,
causes exocrine mucus-producing glands to secrete
an unusually thick mucus that clogs ducts and
collects in lungs and other body areas.
CF patients usually die before the age of 20, while
some individuals live to the age of 30.
Duchenne muscular dystrophy
(DMD)
1. Generalized weakness and muscle wasting
affecting limb and trunk muscles first.
Calves often enlarged. Wheels at 12 y.o.
X-linked recessive disorder; 1/3500 boys worldwide
About 30% of cases represent new mutations.
Life threatening dysrhythmia or heart failure
develops in about 10 %.
Absence of dystrophin, a cell membrane protein
(approximately 0.01 % of skeletal muscle protein).
All muscles involved
Death ay 10
th
-20
th
after pulmonary problems (breathing)
Dystrophin
Provide links between
the intracellular cytoskeleton
and the actin filaments
with the extracellular matrix
Duchenne and Becker MDs
Sarcoglicans:
Limb Girdle MDs (4 types)
Laminin22:
congenital MD chr 6
Whole complex
stabilizes the membrane.
Clinical Examination on identification of areas of muscle
strength and weakness and the progression of weakness
Family History/Genetic Testing
Nerve Conduction and Electromyogram
Serum Enzyme Tests muscle mass may be so reduced that
serum protein levels may even appear normal. Tests routinely
used for diagnosis include creatine kinase, aldolase, lactic
dehydrogenase (LDH), glutamic oxaloacetic transaminase
(GOT) levels, pyruvate kinase (PK)
Muscle Biopsy
- FRAGILE X SYNDROMES
(Fragile Xq or fra(X)(q28))
. Epidemiology:
FRAXA: 0.2 / 1 000 male births and 0.1 / 1 000 female births.
FRAXE: 0.02/ 1 000 male births.
II. Clinics:
the face reminds of the one found in trisomy 8.
macrocephaly.
high forehead.
midface hypoplasia.
large nasal root.
prognathism.
thick lips.
high palate.
large, unfolded ears.
macroorchidy.
fertility is often normal.
Mental development and psychiatric behaviour
In the male:
Mental retardation is mild to severe (mean IQ = 50): from a delay in school training
to the impossibility to acquire writing and reading skills. The Fragile Xq young child
is often hypotonic; in the more severe forms, a psychomotor delay is already
present (delay in walking...).
Speech difficulties: delay language appearance, dysarthria, omissions, mumblings,
echolalias (tendency to repeat the same sentences and to ask the same questions).
Behaviour problems: anguish, attention deficit, hyperactivity, impulsiveness, escape
of glance, resistance to change, aggressiveness, self-mutilation, stereotypies (wings
beating, "flapping") and oddities. Sometimes all these symptoms are present, and
constitute an autistic syndrome.
The various studies carried out among the autists show that 5 to 7 % autists are
Fragile Xq.
In the female:
- The mental retardation is mild or absent.
- They can present with: school difficulties (less than in the boy), memory
disorders, changing mood, timidity, relational difficulties, and depressive
tendency. These symptoms are often misinterpreted for social causes.
IV. Diagnosis: the karyotype can show recurrent gaps in Xq27-q28;
however, the diagnosis now rely on the molecular study of the genes.
THE PRADER-WILLI SYNDROME
is a complex multisystem disorder characterised by a
variety of clinical features
characterised by hyperphagia, childhood-onset-
obesity, severe muscle hypotonia, a typical facies,
hypogonadism with absence of a pubertal growth
spurt, short stature, small hands and feet and
delayed developmental milestones.
The typical facial features include a small forehead,
almond shaped eyes, micrognathia, a thin upper lip
and down-turned corners of the mouth
a multistage disorder characterised by
three different phases .
1. the hypotonic phase,;
varying degrees of hypotonia during the neonatal period and early infancy, a
weak cry,
hypothermia, hypogenitalism and a poor suck reflex usually necessitating
gavage feeding ].
During the first year, PWS children are defined as friendly, easy going and
affectionate
argumentativeness, anxiety and obsessive compulsive symptoms
.
2. the hyperphagic phase, which usually starts between the ages of one and
two ;
- a voracious appetite, hyperphagia, foraging for food,
early onset of childhood obesity, physical inactivity, decreased pain sensitivity,
disturbed thermoregulation, psychomotor retardation,
speech articulation difficulties and cognitive dysfunction.
Simultaneously, with the change in eating pattern, PWS individuals show
significant maladaptive behavioural and emotional characteristics including
temper tantrums, inappropriate social behaviour, automutilation (skin picking),
stubbornness, mood lability, impulsivity, argumentativeness, anxiety and
obsessive compulsive symptoms.
The third phase adolescence and
adulthood
-dominated by health problems secondary to obesity
- These include scoliosis, dental problems, diabetes mellitus,
hypertension, hypercholesterolemia, osteoporosis
- About 10% of the adolescents and adults develop major
psychiatric problems ranging from severe and agitated
depression to psychotic episodes
-The psychotic episodes in PWS patients have many features
in common including an acute onset, a polymorphous and
fluctuating symptomatology with anxieties, agitation,
abnormal beliefs and auditory hallucinations. These episodes
are classified as acute cycloid psychosis
Dysfunction of the hypothalamus may be the basis of a
number of symptoms in the Prader-Willi syndrome.
The fetal hypothalamus plays a major role in labour and
hypothalamic dysfunction may explain the high proportion of
children born prematurely or postmaturely.
Abnormal LSH-releasing hormones are thought to be
responsible for the decreased levels of sex hormones
resulting in non-descended testes, undersized sex organs,
amenorrhoea and insufficient growth during puberty.
Growth hormone deficiency due to hypothalamic
dysregulation contributes to the abnormal growth pattern,
excess of body fat and deficit of lean body mass with
consequent reduced energy expenditure.
Hypothalamic disturbances cause aberrant control of body
temperature and daytime hypersomnolence. The insatiable
hunger and hyperphagia is probably a consequence of the
decreased number of oxytocine neurones- the putative
satiety neurones in the hypothalamic paraventricular nucleus
Molecular genetics of the Angelman and the Prader-
Willi syndrome
result from loss of paternal or maternal expression, respectively, of genes located
on the human chromosome 15q11-13 region
Different molecular mechanisms leading to this loss of expression have been
identified, including intragenic mutations, uniparental disomy and imprinting
defects:
1. Microdeletions
75% of the PWS patients and 70% of the AS patients have large chromosomal
deletions of +/- 4 Mb of the same chromosomal 15q11-13 region,). In PWS
there is a deletion on the paternally inherited chromosome, while in
Angelman there is a deletion on the maternally inherited chromosome.
2. Single gene mutations in PWS and AS
There are no known PWS patients with a single gene mutation, suggesting that
PWS is a continuous gene syndrome. In 4 % of the cases, Angelman is caused by
mutations in the Ubiquitin ligase gene, UBE3AC
3. Imprinting defects in PWS and AS
IC defects are found in 2 % of the AS cases and in less than 1 % of the PWS cases.
4. Uniparental disomy in PWS and AS
Uniparental disomy occurs in 24% of the PWS patients (maternal disomy) and in 3-
5% of AS patients (paternal disomy). The most likely explanation is trisomy 15
rescue, suggested by the observation of trisomy 15 mosaicism in patients with
unusual PWS manifestations
Penugasan
Setiap SGD membuat materi tentang penyakit dibawah ini :
1. Down sindrome
2. Patau Syndrome
3. Fragile X sindrome
4. Marfan sindrome
5. Deuschene muscular distrophy (DMD)
6. Prader wili syndrome
7. Turner syndrome
8. Klinefelter syndrome
9. Galactosemia
10. Phenyl ketonuria
11. Glycogen storage disease
12. Congenital adrenal hiperplasia
13. CAIS (complete adrenal onsufisiensi syndrome)/PAIS
Penugasan
materi di buat perorangan dan diberi nama di
jilid menjadi satu untuk 1 SGD.
tugas diketik dengan spasi 1,5
Bila ada yang ketahuan copy paste/ mencuplik
dari pekerjaan temannya maka baik yang
mencopy atau yang di copy nilai dibatalkan
Pekerjaan paling lambat di kumpul sebelum
SGD 2 LBM 3
You might also like
- Pre-Reading Genetics DisordersDocument10 pagesPre-Reading Genetics DisordersMya Phone MohNo ratings yet
- Genetics 3Document32 pagesGenetics 3Dipesh KhadkaNo ratings yet
- Common Genetic Disorders ExplainedDocument44 pagesCommon Genetic Disorders ExplainedHamza Ali50% (2)
- Down's Syndrome2Document18 pagesDown's Syndrome2fmarnieNo ratings yet
- Chromosomal and GeneticsDocument61 pagesChromosomal and Geneticssomeone in YoutubeNo ratings yet
- Ayling Kuliah Sindroma Down-HandoutDocument23 pagesAyling Kuliah Sindroma Down-HandoutYuliana LatifNo ratings yet
- Full Notes Clinical GeneticsDocument52 pagesFull Notes Clinical GeneticsArundhathyNo ratings yet
- Clinical Vignette 1Document9 pagesClinical Vignette 1Joanne Alyssa Hernandez LascanoNo ratings yet
- Sindroma Down: DR - Ayling Sanjaya, M.Kes, Spa Fakultas Kedokteran Universitas Wijaya Kusuma SurabayaDocument23 pagesSindroma Down: DR - Ayling Sanjaya, M.Kes, Spa Fakultas Kedokteran Universitas Wijaya Kusuma SurabayaFarihatun NisaNo ratings yet
- 4 The Nursing Role in Genetic Assessment and CounsellingDocument97 pages4 The Nursing Role in Genetic Assessment and CounsellingBryan Lloyd Ballestar RayatNo ratings yet
- Kelainan GenetikDocument36 pagesKelainan GenetikImas Siti MNo ratings yet
- Human Disease Genes or Human Genetic DiseaseDocument23 pagesHuman Disease Genes or Human Genetic Diseasebalkrishn joshiNo ratings yet
- Downsyndrome 150808111351 Lva1 App6892Document20 pagesDownsyndrome 150808111351 Lva1 App6892Mohammad Saadullah Khan KakarNo ratings yet
- Nurse As An Art - OmdDocument67 pagesNurse As An Art - OmdJozarine Chelsea LopezNo ratings yet
- Turner Syndrome, Klinefelter Syndrome, Down SyndromeDocument78 pagesTurner Syndrome, Klinefelter Syndrome, Down SyndromeTasya100% (3)
- Congenital Anomalies: Dr. Bertha Soegiarto, Sp.ADocument26 pagesCongenital Anomalies: Dr. Bertha Soegiarto, Sp.AChristine VerinaNo ratings yet
- Down Syndrome - Copy-1Document56 pagesDown Syndrome - Copy-1Holden CaulfieldNo ratings yet
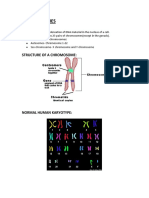
- Chromosomes: Structure of A ChromosomeDocument27 pagesChromosomes: Structure of A ChromosomeLaika LaiNo ratings yet
- Autosomal trisomies overviewDocument39 pagesAutosomal trisomies overviewmada_baiasNo ratings yet
- Genetic Disorders at Haramaya UniversityDocument32 pagesGenetic Disorders at Haramaya UniversityMerwan KemalNo ratings yet
- Chromosomal Disorder: Genetics DiseasesDocument40 pagesChromosomal Disorder: Genetics Diseaseslilik maula shobriNo ratings yet
- Sindroma Down: Fakultas Kedokteran Universitas - Wijaya Kusuma SurabayaDocument23 pagesSindroma Down: Fakultas Kedokteran Universitas - Wijaya Kusuma SurabayaPrinces Mentari Dwi NurainiNo ratings yet
- Kelainan GenetikDocument36 pagesKelainan GenetikNicko Erdy Kusuma100% (1)
- Genetic DisorderDocument13 pagesGenetic DisorderLucreatia RynjahNo ratings yet
- Methods of Human GeneticsDocument81 pagesMethods of Human Geneticsfae-ar_raziNo ratings yet
- Mutations Genetic Disorders 22Document34 pagesMutations Genetic Disorders 22WESLEY ARCONNo ratings yet
- Congenital Anomalies: Pooja K MenonDocument73 pagesCongenital Anomalies: Pooja K Menonpujitha2002100% (2)
- Human GeneticsDocument15 pagesHuman GeneticsKLENNY ADRIA BATISANNo ratings yet
- Chromosomal Disorders SimpleDocument8 pagesChromosomal Disorders SimpleSadia KanwalNo ratings yet
- Genetic Counseling in DentistryDocument8 pagesGenetic Counseling in Dentistrykhaled alahmadNo ratings yet
- Down Syndrome Diagnosis, Risk Factors and ManagementDocument74 pagesDown Syndrome Diagnosis, Risk Factors and Managementanumeha sharma100% (2)
- 5chromosomalabnormalities (L5)Document45 pages5chromosomalabnormalities (L5)d.djumanalieva.97No ratings yet
- Pisharody Genetic CounselingDocument15 pagesPisharody Genetic Counselingapi-228136529No ratings yet
- Types of MutationDocument11 pagesTypes of MutationNitesh ShirurNo ratings yet
- Anatomy Structural Numerical Abnormalities of Cell DivisionDocument52 pagesAnatomy Structural Numerical Abnormalities of Cell Divisionhabibaatif10No ratings yet
- Biology Project: Name Ajit Kumar CLASS 12 S2Document7 pagesBiology Project: Name Ajit Kumar CLASS 12 S2ajitNo ratings yet
- Genetic TestingDocument18 pagesGenetic Testingneha100% (1)
- Pediatrics Genetics and SyndromesDocument24 pagesPediatrics Genetics and Syndromesyoune6No ratings yet
- 3 - Genetics Lec.3 (Gross Structural Anomalies of Chromosoms)Document27 pages3 - Genetics Lec.3 (Gross Structural Anomalies of Chromosoms)Ammar AlnajjarNo ratings yet
- Framework For Maternal and Child Health NursingDocument147 pagesFramework For Maternal and Child Health NursingRouie Björn ABrianNo ratings yet
- Genetics Lecture SummaryDocument52 pagesGenetics Lecture Summarykoalakid55No ratings yet
- SCIENCES GENE REPORT JskaDocument4 pagesSCIENCES GENE REPORT Jskaheart louvNo ratings yet
- Congenital and Hereditary Diseases Introduction-1Document21 pagesCongenital and Hereditary Diseases Introduction-1Melvin OnyanchaNo ratings yet
- Genes - Are The Basic Units of Heredity That Determine Both Chromosomes - These Are The Gene Strands in The Nucleus of All 46XYDocument13 pagesGenes - Are The Basic Units of Heredity That Determine Both Chromosomes - These Are The Gene Strands in The Nucleus of All 46XYMilo AlmirolNo ratings yet
- Ana 231 Chromosome AnomaliesDocument28 pagesAna 231 Chromosome AnomaliesAmadi CedarNo ratings yet
- AneuploidyDocument4 pagesAneuploidyTingal, Jaynore C.No ratings yet
- Down SyndromeDocument20 pagesDown Syndromebarshanandi82No ratings yet
- Common Physical and Mental Characteristics of Down SyndromeDocument9 pagesCommon Physical and Mental Characteristics of Down SyndromeKhaled Zeama100% (1)
- Congenital & Hereditary Diseases Seminar (BIY5006Document18 pagesCongenital & Hereditary Diseases Seminar (BIY5006chelsea charlesNo ratings yet
- Human Genetic Disorders: A Visual GuideDocument49 pagesHuman Genetic Disorders: A Visual GuideJessie AzarragaNo ratings yet
- Genetic Disorders-1Document42 pagesGenetic Disorders-1Bala UrmarNo ratings yet
- Down Syndrome - Group 1 (Roll No 1-22)Document26 pagesDown Syndrome - Group 1 (Roll No 1-22)Kaung Wai Yan HeinNo ratings yet
- Pediatric Genetics Dr. Samed AlsalmiDocument166 pagesPediatric Genetics Dr. Samed AlsalmiabhivnairNo ratings yet
- GeneticsDocument154 pagesGeneticsNooneNo ratings yet
- Definition: Down Syndrome, Down's Syndrome, or Trisomy 21 Is A Chromosomal Disorder Caused by TheDocument3 pagesDefinition: Down Syndrome, Down's Syndrome, or Trisomy 21 Is A Chromosomal Disorder Caused by TheLove Shery SabrosoNo ratings yet
- Kuliah Congenital Abnormalities PediatricDocument27 pagesKuliah Congenital Abnormalities Pediatricmuhammad alqoriNo ratings yet
- Autosomal Inheritance Patterns & Genetic Disorders (40Document45 pagesAutosomal Inheritance Patterns & Genetic Disorders (40Mary Grace SunioNo ratings yet
- Patterns: OF InheritanceDocument45 pagesPatterns: OF InheritanceMary Grace SunioNo ratings yet
- ID Perancangan Sistem Pembayaran Billing System Pasien Di Rumah Sakit Umum Inanta P PDFDocument7 pagesID Perancangan Sistem Pembayaran Billing System Pasien Di Rumah Sakit Umum Inanta P PDFFahmi Nur ZamanNo ratings yet
- ID Perancangan Sistem Pembayaran Billing System Pasien Di Rumah Sakit Umum Inanta P PDFDocument7 pagesID Perancangan Sistem Pembayaran Billing System Pasien Di Rumah Sakit Umum Inanta P PDFFahmi Nur ZamanNo ratings yet
- MorbiliDiagnosisDocument6 pagesMorbiliDiagnosisFrans SikhaNo ratings yet
- Devada Ayu Permatasari: Fresh GradDocument1 pageDevada Ayu Permatasari: Fresh GradFahmi Nur ZamanNo ratings yet
- Postmortem Biochemistry of Vitreous Humor and Glucose MetabolismDocument7 pagesPostmortem Biochemistry of Vitreous Humor and Glucose MetabolismsbeyeforhireNo ratings yet
- Postmortem Biochemistry of Vitreous Humor and Glucose MetabolismDocument7 pagesPostmortem Biochemistry of Vitreous Humor and Glucose MetabolismsbeyeforhireNo ratings yet
- Circulating Microparticles in Preeclampsia: Research ArticleDocument4 pagesCirculating Microparticles in Preeclampsia: Research ArticleFahmi Nur ZamanNo ratings yet
- ASTHENOPIADocument12 pagesASTHENOPIAIntan Elok PermataSariNo ratings yet
- Neuro Ge NikDocument3 pagesNeuro Ge NikFahmi Nur ZamanNo ratings yet
- JADWAL OP8juliDocument10 pagesJADWAL OP8juliFahmi Nur ZamanNo ratings yet
- The APACHE II Severity of Disease Classification SystemDocument2 pagesThe APACHE II Severity of Disease Classification SystemFahmi Nur ZamanNo ratings yet
- Data Pasien Periode Dokter BetaDocument14 pagesData Pasien Periode Dokter BetaFahmi Nur ZamanNo ratings yet
- GDFGR ADocument1 pageGDFGR ASelvi AnnisaNo ratings yet
- Drug Delivery Devices PDFDocument20 pagesDrug Delivery Devices PDFAnhy OmmyNo ratings yet