Professional Documents
Culture Documents
Short Bowel Syndrome Guide
Uploaded by
bencleeseOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Short Bowel Syndrome Guide
Uploaded by
bencleeseCopyright:
Available Formats
Short Bowel
Syndrome
Riad M. Rahhal
CHAPTER 20
DEFINITIONS AND EPIDEMIOLOGY
Short bowel syndrome (SBS) is a disorder of malab-
sorption resulting from signicant small bowel loss sec-
ondary to congenital disease or surgical resection. The
incidence of SBS is estimated at 1200 per 100,000 live
births.
1
SBS is the most common cause of intestinal fail-
ure. This is defined as a significant reduction in
functional small bowel mass, leading to inadequate
digestion and absorption, with subsequent growth
failure. Other less common causes of intestinal failure
include structural enterocyte defects and severe disorders
of intestinal motility.
2
At birth, the estimated small bowel length is
approximately 250 cm for term or near-term infants
and approximately 100120 cm for premature infants
30 weeks gestation. Small bowel length is thought to
double in the last trimester of pregnancy. Different sys-
tems have been used to describe SBS including those
based on etiology, age, and anatomical considerations.
In the context of surgical resection, the remaining small
bowel length is often used to categorize disease severity.
In a short resection, 100150 cm of small bowel
remains, compared to 40100 cm with a large resection
and 40 cm remaining after a massive resection.
2
The small bowel has considerable adaptive
capacity to compensate for intestinal loss. Intestinal
adaptation is dened as a growth process of the remain-
ing small bowel, through morphological and functional
changes, leading to improved absorption. This process
starts shortly after bowel loss but often continues for
months to years. Depending on the segment and length
of the lost small bowel, patients with SBS are frequently
dependent on parenteral nutrition for prolonged peri-
ods of time, sometimes indenitely. The use of parenteral
nutrition has signicantly improved the life expectancy
of children with SBS. On the other hand, long-term
parenteral nutrition can be associated with a variety of
complications, some of which can have grave
consequences. Patients with SBS who fail medical ther-
apy or develop complications may require surgical
interventions, including transplantation. Because of the
relatively high mortality, SBS is considered among the
most lethal disorders in young children.
3
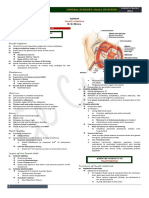
PATHOGENESIS
The small bowel is a vital organ involved in digestion,
absorption, and uid balance. The small bowel is divided
into three anatomical segments (Figure 201). The rst
is the duodenum, which extends from the pylorus to the
duodenojejunal junction, dened by the ligament of
Treitz. The proximal half of the remaining small intes-
tine is composed of jejunum and the distal portion is
ileum. Some absorption takes place in the duodenum,
Duoodenum
Transverse
colon
Ascending
colon
Cecum
Ileocecal
valve
Rectosigmoid R
olon co
Descending
colon
Jejunum
Ileum
Duodeno
jejunal
junction
FIGURE 201 Normal gastrointestinal anatomy.
Bishop_Ch20_295-305.indd 295 4/6/10 4:14:40 PM
296 Section 3: Disorders of the Stomach and Intestine
including that of iron. The main function of the duode-
num is to neutralize acidic gastric contents and mix
them with intestinal, pancreatic, and hepatic digestive
secretions. Absorption mostly takes place in the jeju-
num and ileum. The jejunum has an abundant surface
area enhanced by folds and numerous tall villi. The
luminal surface of enterocytes, or intestinal epithelial
cells, is in turn covered with nger-like projections
termed microvilli, which are collectively referred to as
the brush border. Villi become shorter and less abundant
in the ileum, which therefore has less absorptive surface
area than the jejunum. The center of each villus is occu-
pied by a capillary network that absorbs nutrients which
are eventually transported to the liver by the portal
venous system. The terminal ileum has a high
concentration of lymphoid tissue that assists in immune
regulation. The terminal ileum also has site-specic
receptors for absorption of bile acids and vitamin B
12
.
The ileocecal valve, separating the ileum from the prox-
imal colon, slows the movement of uid into the cecum
and limits bacterial migration from the colon into the
small bowel.
4
During embryonal development, the small intes-
tine arises from the midgut, which extends from the
mid-duodenum to the distal transverse colon. The
duodenum has an extensive blood supply derived from
the celiac axis and the superior mesenteric artery. This
dual blood supply helps avoid ischemia if the supply
from one of the major vessels becomes compromised.
The remainder of the midgut, extending from the jeju-
num to the proximal two-thirds of the transverse colon,
depends on blood supply from branches of the supe-
rior mesenteric artery. Therefore, extensive bowel loss
can result from compromise of the superior mesen-
teric arterial blood ow. The midgut drains through
the superior mesenteric vein, which joins the splenic
vein in forming the portal vein.
4
About 7080% of
blood entering the liver is venous blood from the por-
tal vein, and the remainder is arterial blood supplied by
the hepatic artery. This is important as small bowel
disease can have a signicant negative impact on the
liver with possible bacterial translocation and liver
dysfunction.
Major physiologic disturbances can result from
loss of large sections of the small bowel absorptive
surface area. Consequences especially include malab-
sorption of uid, electrolytes, macronutrients (pro-
teins, carbohydrates, and fats), and micronutrients
(vitamins and trace elements). In general, jejunal
resections are better tolerated than is loss of ileal seg-
ments. When a signicant portion of jejunum is lost,
intestinal adaptation of the ileum can compensate for
many of the jejunal functions. Certain jejunal func-
tions, however, cannot be replaced, including loss of
enteric hormone production (such as cholecystokinin
and motilin) that affects intestinal motility and diges-
tion. Loss of jejunum is often accompanied by decreas-
ing biliary and pancreatic secretions and increased
gastrin levels, leading to gastric hypersecretion. Major
ileal resections can reduce absorption of uid, elec-
trolytes, bile acids, and vitamin B
12
. These functions
cannot be taken over by the remaining jejunum. Bile
acids, excreted into the duodenum by the liver, are
required for the absorption of long-chain fatty acids
and fat-soluble vitamins in the ileum. Loss of bile
acids may therefore contribute to diarrhea by worsen-
ing fat malabsorption. Vitamin B
12
binds to intrinsic
factor, which is produced in the stomach, and the
complex is subsequently absorbed in the terminal
ileum. Bile acid and vitamin B
12
absorption cannot be
replaced by the jejunum. The ileocecal valve plays an
important role in slowing intestinal transit to allow
more time for absorption. The absence of the ileoce-
cal valve, in addition to reducing transit time, can also
lead to small bowel bacterial overgrowth (SBBO) from
colonization by colonic bacteria. SBBO can result in
mucosal injury and worsening diarrhea.
5
The colon, if
present, can play an important role in absorbing
water, sodium, and short-chain fatty acids in SBS
patients. A summary of possible consequences of
loss of specic intestinal segments is presented in
Table 201.
In children, the causes of SBS are variable and can
be classied by the age at the time of intestinal loss
(Table 202).
3,5,6
The outcome will depend on the length
and functionality of the remaining small bowel. Other
contributing factors include the presence or absence of
the ileocecal valve and colon, and associated complica-
tions, especially liver disease.
Possible Consequences of Specic Intestinal
Resections
Loss of duodenum
Iron malabsorption
Loss of jejunum
Calcium malabsorption
Folate malabsorption
Fat-soluble vitamin malabsorption
Gastric hypersecretion
Loss of ileum
Bile acid malabsorption
Vitamin B
12
malabsorption
Loss of the ileocecal valve
Small bowel bacterial overgrowth
Increased uid and electrolyte losses
Table 201.
Bishop_Ch20_295-305.indd 296 4/6/10 4:14:49 PM
CHAPTER 20 Short Bowel Syndrome 297
CLINICAL PRESENTATION
History
A thorough review of the past medical and surgical his-
tory is essential. Children with SBS often have an obvi-
ous event or events that lead to bowel loss. Frequently
noted causes include a major congenital anomaly such
as gastroschisis or intestinal atresia, or the occurrence of
an abdominal catastrophe such as midgut volvulus or
extensive bowel resection after necrotizing enterocolitis
(Table 202). Occasionally, patients may undergo large
or multiple intestinal resections from Crohns disease or
extensive Hirschsprungs disease. Inquiring about the
underlying etiology of SBS is important, because it can
shed light on the portion of small bowel affected and
the functionality of the remaining bowel. Other impor-
tant historical aspects include the remaining length and
segment of small bowel, the presence of an ileocecal
valve and colon, and the continuity of the remaining
bowel (versus presence of ostomies). These can be based
on a thorough review of the surgical records and direct
discussions with the surgical service.
It is critical to perform a thorough nutritional
assessment. History of previous and current nutritional
intake and tolerance should be obtained. Patients with
SBS are typically on total or near-total parenteral nutri-
tion through a central venous line. Most are receiving
little to no enteral nutrition shortly after the event that
leads to bowel loss. For parenteral nutrition, the amount
of intravenous dextrose, amino acids, and lipids as well
as the electrolyte, multivitamin, and trace element solu-
tions should be determined. For enteral nutrition, the
type (breast milk versus formula), caloric density, total
volume, and mode (continuous versus bolus, oral versus
tube feeding) of feedings should be known. Caloric
intake should be assessed separately for parenteral and
enteral nutrition. The aim of therapy is to gradually
increase the caloric intake from enteral nutrition and
decrease that from the parenteral route while maintain-
ing appropriate growth and development.
Previous and current central line access should be
reviewed. Any previous history of central line thrombo-
sis or infection should be obtained, including causative
organisms and related clinical complications. Frequent
line infections requiring line changes can raise concern
about line care quality, intestinal bacterial translocation,
worsening-associated liver disease, and limited long-
term vascular access. This complication can lead to
adjustments in central line care and the feeding regi-
men, as well as earlier consideration for other surgical
interventions, including transplantation.
The clinical symptoms associated with SBS can
vary but often include diarrhea, weight loss or poor
growth, fatigue, and lethargy. These symptoms arise
from underlying dehydration, electrolyte abnormalities,
and calorie and nutrient deciencies. Stool output will
be higher in children with SBS because of limited
absorptive surface even with only partial enteral feed-
ing. Worsening diarrhea can imply reaching or going
beyond the maximum absorptive capacity of the remain-
ing bowel. Diarrhea can also be seen with SBBO, which
is common among SBS patients. A history of recurrent
abdominal distention, foul-smelling stools, and atu-
lence should alert the managing physician to possible
SBBO, especially when the ileocecal valve has been
resected, allowing retrograde contamination of the
small intestine with colonic contents. Weight loss or
poor growth suggests inadequate caloric intake, and
should lead to adjustments in the nutritional manage-
ment. Certain vitamin and other micronutrient de-
ciencies can lead to specic symptoms that should be
recognized and treated promptly (see Table 86).
Liver disease can affect 4060% of infants receiv-
ing prolonged parenteral nutrition. The presence of
liver disease, especially cholestasis, signicantly worsens
the outcome of children with SBS.
7
The history should
therefore include an assessment for possible liver disease
and related complications including portal hyperten-
sion, history of gastrointestinal bleeding, and signs of
fat malabsorption.
PHYSICAL EXAMINATION
Accurate weight and height measurements are needed.
The presence of temporal wasting, loss of muscle mass
and subcutaneous fat, poor dentition, and peripheral
edema suggest severe protein and energy malnutrition.
Causes of Short Bowel Syndrome in Children
Prenatal/neonatal period
Intestinal atresia
Midgut or segmental volvulus
Gastroschisis
Omphalocele
Necrotizing enterocolitis
Extensive Hirschsprungs following resection
Postnatal period
Midgut volvulus
Mesenteric infarction
Trauma
Crohns disease requiring resection
Radiation enteritis
Intestinal tumor
Table 202.
Bishop_Ch20_295-305.indd 297 4/6/10 4:14:49 PM
298 Section 3: Disorders of the Stomach and Intestine
Other general features of malnutrition include dry skin,
prominent nail ridges, and blunted lingual papillae.
Patients can have physical ndings and symptoms
related to specic micronutrient deciencies. Essential
fatty acid deficiency (linoleic acid and linolenic acid)
may occur, manifesting as growth retardation, dermati-
tis, and alopecia. Patients with SBS and cholestasis
should be closely monitored clinically and biochemi-
cally for fat-soluble vitamin deciency (A, D, E, and K).
Vitamin A deficiency is associated with signicant ocular
impairment. Vitamin A absorption and metabolism can
also be impaired by underlying zinc deciency. Signs of
zinc deciency include poor growth and wound healing,
diarrhea, alopecia, and angular stomatitis. Manifesta-
tions of vitamin E deficiency can include ocular palsy,
wide-based gait, and decreased deep tendon reexes.
The presence of ecchymoses, purpura, or bleeding
diatheses should raise concern about vitamin K de-
ciency. Vitamin D deficiency is associated with rickets
and osteomalacia. Biochemically, vitamin D-decient
patients can have hypocalcemia, hypophosphatemia,
and elevated alkaline phosphatase levels.
Patients with signicant resections of the distal
ileum are at high risk of developing vitamin B
12
defi-
ciency. Deciency in vitamin B
12
(cobalamin) can result
in megaloblastic anemia and demyelination. Folic acid
deficiency can lead to megaloblastic anemia, neutrope-
nia, and impaired growth. Iron deciency can present
with pallor, fatigue, spooned nails, and glossitis. In gen-
eral, micronutrient deciencies are uncommon occur-
rences with parenteral nutrition and are more likely to
develop once parenteral nutrition is discontinued, as
intestinal absorption may be suboptimal.
Physical signs of associated liver disease can
include jaundice, scleral icterus, excoriations secondary
to pruritis, splenomegaly, ascites, and poor growth.
Abdominal distention and tympany may suggest SBBO
from colonic bacterial translocation into the small bowel
or from intestinal strictures following surgery.
DIFFERENTIAL DIAGNOSIS
Because children with SBS usually have a specic event
leading to bowel loss or resection, the diagnosis is rarely
confused with other conditions. However, some patients
may have a long history of multiple enterectomies leading
eventually to SBS, such as patients with small bowel
Crohns disease. Common symptoms in SBS patients,
such as diarrhea and lack of growth, can be exacerbated
by other factors, including gastrointestinal and extraintes-
tinal infections, SBBO, intestinal strictures, loss of bowel
function (despite reasonable remaining bowel length),
dysmotility, and Munchausen by proxy. These possibili-
ties should be investigated and addressed appropriately.
DIAGNOSIS
The diagnosis of SBS can be conrmed based on the
surgical records that indicate the scope of bowel resec-
tion and the length of the remaining small bowel. Upper
GI and small bowel contrast imaging can assist in deter-
mining the extent of the remaining bowel. Occasionally
endoscopic evaluation with esophagogastroduodenos-
copy and colonoscopy may help dene the anatomy of
the remaining bowel. The serum level of citrulline, an
amino acid mainly produced by enterocytes, has been
used as a quantitative biomarker to assess the remaining
functional absorptive capacity. Reduced plasma citrul-
line levels are suggestive of intestinal failure.
8
A level
19 mmol/L has been postulated to predict achieving
enteral autonomy in children with SBS.
9
Clinically, fail-
ure to tolerate enteral nutrition, with diarrhea and
growth retardation despite having near-normal bowel
length, may indicate dysfunction of the present bowel
with intestinal failure.
Associated liver disease should be suspected based
on biochemical tests including bilirubin and transami-
nase levels. Cholestasis is dened as a conjugated bili-
rubin level that is 20% of the total bilirubin. Many
studies have used a conjugated bilirubin level of 2
mg/dL to report on parenteral nutrition-associated
cholestasis. Hepatic synthetic function can be evaluated
by serum albumin and prothrombin time, although
these tests can be affected by the overall nutritional sta-
tus. The gold standard for diagnosing liver disease is by
liver biopsy. Histologic liver abnormalities can vary and
include canalicular and intralobular cholestasis, steato-
sis, periportal inammation, bile duct proliferation,
portal brosis, and cirrhosis.
TREATMENT
Immediately after bowel loss, total parenteral nutrition
is required until bowel function returns. The main
challenge shortly after bowel resection is maintaining
proper uid balance. Once signicant uid and electro-
lyte losses are reduced, enteral feedings should be initi-
ated. Depending on the severity, full enteral nutrition
may be achieved within weeks to months. However, in
some cases it is never achieved. It is important that
every patient be given as much enteral nutrition as pos-
sible to facilitate the process of intestinal adaptation
that leads to bowel growth and increased nutrient
absorption, and diminishes the potentially hepatotoxic
effects of parenteral nutrition.
Treatment of children with SBS involves a multi-
disciplinary approach engaging the pediatrician or fam-
ily physician and an experienced center with pediatric
gastroenterologists, nutritionists, speech and behavioral
Bishop_Ch20_295-305.indd 298 4/6/10 4:14:49 PM
CHAPTER 20 Short Bowel Syndrome 299
therapists, pediatric surgeons, and care coordinators.
Insuring psychosocial support for the child and the
family is essential. Proper education of the family about
the management and expectations during the different
phases of care should be provided.
Venous Nutrition
Parenteral nutrition is the mainstay of treatment for
SBS. Its components include carbohydrate, protein, lip-
ids, electrolytes, vitamins, and trace minerals. Parenteral
nutrition should be individualized based on the childs
caloric needs, nutritional status, and laboratory testing.
The assistance of a dietitian and pharmacist can be vital
in the management of parenteral nutrition. Estimated
energy requirements by age are provided in Table 84.
Most children may have been already on parenteral
nutrition prior to the resection. Table 203 provides
formulas to assist with calculations for components of
parenteral nutrition. For patients who have not been
started on parenteral nutrition, the following section
may provide some guidelines.
The carbohydrate component is supplied as dex-
trose. Most infants will be euglycemic on an initial glu-
cose infusion rate (GIR) of 58 mg/kg/minute that can
be gradually increased to about 14 mg/kg/minute. A
GIR of 1214 mg/kg/minute is usually well tolerated in
older children. Infusion rates in excess of 20 mg/kg/
minute can promote fatty liver inltration and should
be avoided. A dextrose 12.5% solution should not be
exceeded when using a peripheral intravenous line.
With central lines, dextrose solutions as high as 25%
can be gradually attained. Advancement in dextrose
concentration by 2.5% increments per day in infants
and 5% increments per day in older children is gener-
ally well tolerated. Monitoring serum glucose levels
with changing dextrose concentrations and/or infusion
rates is important to identify and address signicant
uctuations.
In the young infant, amino acid content can be
started at 0.51 g/kg/day and then increased by incre-
ments of 0.5 g/kg/day to a target of 2.53 g/kg/day. In
older children, amino acid content can be provided
directly at the intended goal.
Intravenous lipids have the highest caloric density
in parenteral nutrition and are important to avoid
essential fatty acid deciency when enteral intake is very
limited. In infants, intravenous lipids can be started at
0.5 g/kg/day using a 20% solution and then increased by
increments of 0.5 g/kg/day to a maximum of 3 g/kg/day.
In older children, initial infusions at 1 g/kg/day can be
used with target goals depending on age. Typically no
more than 3040% of total daily calories should come
from lipids. Serum triglycerides should be monitored to
insure lipid clearance from the circulation. Serum trig-
lyceride levels are best checked 4 hours after the start of
the fat infusion. Reductions in intravenous lipid deliv-
ery should be considered if serum triglyceride levels
approach 250 mg/dL. Providing intravenous lipids over
20 hours/day has also been suggested to facilitate lipid
clearance.
A pediatric formulation of trace elements should
be provided in parenteral nutrition that includes zinc,
copper, manganese, chromium, and selenium. Levels of
these trace elements should be monitored. In the setting
of established cholestasis, increased serum copper and
manganese levels should be addressed as they are pri-
marily liver excreted and may worsen liver disease. This
should include a reduction in the copper content and an
elimination of manganese from parenteral nutrition.
10
If removed or reduced, serum copper levels should still
be followed to avoid copper deciency that can lead to
neutropenia and hypochromic anemia.
11
Monitoring
for zinc deciency is important; however, the optimal
test remains unknown. Serum zinc only measures albu-
min-bound zinc and may not accurately assess total-
body stores. In the setting of a low or low-normal serum
zinc level and a low alkaline phosphatase level, zinc de-
ciency should be seriously considered and supplemen-
tation, either parenteral or enteral, should be started.
10
Complications related to the use and changes in
parenteral nutrition should be monitored including
hypo-or hyperglycemia, electrolyte abnormalities,
refeeding syndrome, and liver disease. Central line com-
plications can be associated with signicant morbidity
and mortality and include infections, phlebitis, throm-
bosis, pulmonary embolism, and line occlusion.
Suspected line infections should be treated aggressively
with intravenous antibiotics until blood culture results
with organism susceptibilities are available. The onset of
fever, worsening cholestasis, hyperglycemia, acidosis,
Calculations for Parenteral Nutrition Components
Macronutrient content
GIR (mg/kg/minute) = [(% dextrose solution) (total volume,
mL)]/[(weight, kg) 144]
Intravenous protein intake (g/kg/day) = [(% amino acid
solution) (total volume, mL)]/[100 (weight, kg)]
Intravenous lipid intake (g/kg/day) = [(% lipid solution)
(total volume, mL)]/[100 (weight, kg)]
Caloric intake
Calories from non-lipid components: [(3.4 % dextrose
solution) (4 % amino acid solution)]/100
Calories from lipid component: 2 kcal/mL for 20% lipid
solution
Table 203.
Bishop_Ch20_295-305.indd 299 4/6/10 4:14:49 PM
300 Section 3: Disorders of the Stomach and Intestine
thrombocytopenia, and feeding intolerance should raise
suspicion for a possible central line infection. Central
lines should be handled with an aseptic technique to
avoid infection. Lines impregnated with antibiotics or
silver, or the use of antibiotic or ethanol locks, have been
proposed to reduce bacterial colonization or treat per-
sistent line infections.
12,13
As enteral nutrition is
advanced, parenteral nutrition should be weaned with
the ultimate goal of total discontinuation and central
line removal.
Enteral Nutrition
Enteral nutrition should be initiated as soon as possible
to start the process of intestinal adaptation, to stimulate
gastrointestinal secretions and enhance production of
trophic hormones. When enteral feeds are started,
cycling parenteral nutrition over 1220 hours may help
alleviate liver dysfunction and allow more freedom for
the child and family. The initiation and advancement of
enteral nutrition by itself plays a pivotal role in reducing
the frequency of associated liver disease.
There is no consensus on the optimal type or
mode of enteral nutrition. Lactose-free formulas are
usually preferred to avoid consequences of lactose mal-
absorption. Many centers utilize hydrolyzed or amino
acid-based formulas to enhance intestinal absorption
and address concerns about antigenic load related to gut
inammation and disruption in the mucosal barrier.
14
On the other hand, other centers use breast milk or
polymeric formulas. Breast milk can provide trophic
factors that promote intestinal growth. Use of polymeric
formulas may enhance intestinal adaptation more than
elemental formulas. Medium-chain triglyceride (MCT)-
rich formulas or supplements are commonly used in the
setting of cholestasis to enhance fat absorption and
improve growth. MCTs can be better absorbed because
they are not dependent on bile ow. It is important to
note that MCTs do not provide essential fatty acids, so
prolonged use of exclusive MCT formulas may lead to
essential fatty acid deciency.
Continuous tube feeds (nasogastric or gastros-
tomy) are often utilized initially. This allows for con-
tinuous saturation of the intestinal transporters to
enhance absorption.
15
Subsequently, feeds can be
adjusted to allow frequent small-volume boluses (orally
or by feeding tube) during the day with continuous tube
feeds at night. In general, enteral feeds are started using
a non-concentrated formula given at a low rate. The
caloric density and formula volume can be gradually
advanced depending on feeding tolerance and stool
output. Many children with SBS may have feeding dif-
culties with oral aversion and would benet from early
consultation with a behavioral feeding specialist. Even if
a continuous feeding regimen is chosen, an oral
stimulation program should be instituted early. Trails
with age-appropriate solid food orally should not be
withheld. Beverages rich in simple carbohydrates (such
as juices) should be limited and preferably eliminated to
avoid osmotic diarrhea.
16
As enteral feeds are being advanced, children with
SBS should be monitored for increasing stool output,
dehydration, electrolyte abnormalities, and perineal
dermatitis. Although there is no consensus on the
acceptable upper limit of stool output, many centers use
4050 mL/kg/day. Other commonly used contraindica-
tions to advancement of enteral nutrition include an
increase in stool output by 50% in 24 hours, stool
pH 5.5, and strongly positive stool test for reducing
substances.
17
Fecal-reducing substances 1% and fecal
pH 5.5 usually indicate severe carbohydrate malab-
sorption. The presence of signicant perineal dermatitis
may also be a sign of a very high stool output.
Micronutrients
Fat malabsorption may be a signicant problem in SBS
patients with cholestasis. Supplementation with fat-sol-
uble vitamins is needed to avoid or treat deciencies
associated with impaired fat absorption. Vitamin de-
ciency is not usually a problem when receiving paren-
teral nutrition as these vitamins are delivered directly
into the bloodstream. Once a child with SBS has reached
full enteral feeds and is off parenteral nutrition, ade-
quacy of micronutrient absorption becomes a concern,
so levels of vitamins, iron, magnesium, and zinc should
be followed. Recommendations on micronutrient sup-
plementation are presented in Table 816. Further dose
adjustments may be needed based on serum vitamin
levels. Fat-soluble vitamins can be continued for about
3 months after resolution of cholestasis, as normal bile
ow may be delayed. Children who have lost a substan-
tial portion of their distal ileum will require vitamin B
12
supplementation. It can take several years for a vitamin
B
12
deciency to develop, so regular long-term monitor-
ing is needed. Appropriate vitamin B
12
levels can be
achieved by monthly injections. Other modes of vita-
min B
12
supplementation include intranasal applica-
tions and high oral doses. In humans, the essential fatty
acids are linoleic acid and linolenic acid. In essential
fatty acid deciency, the trienoic:tetraenoic ratio can be
measured, usually rising above 0.2. Normally, essential
fatty acid deciency can be avoided by providing pre-
mature infants with 0.60.8 g/kg/day and older children
with 0.51 g/kg/day of intravenous lipids.
Supplements that may enhance intestinal adapta-
tion (glutamine, growth hormone, and glycogen-like
peptide) or slow intestinal transit (increased fiber
intake) have been studied. Their benets have not been
conrmed, so they are occasionally used.
3
Bishop_Ch20_295-305.indd 300 4/6/10 4:14:49 PM
CHAPTER 20 Short Bowel Syndrome 301
Diarrhea
Diarrhea occurs commonly in SBS patients and can be
from a combination of increased secretions, increased
motility, and malabsorption. Other contributing factors
include SBBO syndrome and gastric hypersecretion.
Diarrhea can be initially controlled by restricting enteral
intake, which reduces the osmotic component. Gastric
hypersecretion can be addressed by use of an H2 recep-
tor antagonist or a proton pump inhibitor, especially in
the rst few months after intestinal resection. Loper-
amide hydrochloride (Imodium) may be used to
decrease stool frequency. Codeine and diphenoxylate
hydrochloride (Lomotil) may also help slow the intesti-
nal motility, but can be associated with side effects,
including addiction, and can worsen bacterial over-
growth. Cholestyramine (Questran), a bile salt-binding
resin, can be used to bind bile salts in patients with bile
salt malabsorption.
Small Bowel Bacterial Overgrowth
As mentioned above, SBBO should be suspected in
patients with worsening diarrhea, foul-smelling stools,
atulence, abdominal distention, and cramps. The diag-
nosis is often made based on clinical grounds and
response to enteral antibiotic therapy. Conrmation
(which is seldom necessary) requires a quantitative cul-
ture of aspirated small bowel uid. The presence of
10
5
colony-forming units of non-pharyngeal bacteria
on culture is supportive of the diagnosis. Treatment of
SBBO can resolve the presenting symptoms and also
minimize bacterial translocation across the intestinal
mucosa and resultant bacteremia. Antibiotics are often
given in cycles of 510 days at the beginning of every
month. In some patients, continuous alternating antibi-
otic cycles may be necessary to avoid development of
resistant bacteria. Antibiotic choices include enteral
metronidazole, gentamicin, neomycin, rifaximin, and
trimethoprim-sulfamethoxazole.
Associated Liver Disease
Prolonged use of parenteral nutrition has been impli-
cated in the development of cholelithiasis, cholestasis,
and steatosis. Transient elevations of liver tests can be
observed early with parenteral nutrition; however, with
prolonged use, signicant liver dysfunction can occur,
with cholestasis progressing to cirrhosis and liver
failure. Risk factors predisposing SBS patients to liver
dysfunction include prolonged use of parenteral nutri-
tion, reduced intestinal length, bacterial overgrowth,
and interruption of the intrahepatic circulation after
ileal resection. Other factors may include manganese
and copper toxicity. Both of these metal ions are mainly
eliminated with bile and can accumulate in the liver
during cholestasis, possibly contributing to the liver
injury.
A conjugated bilirubin level 2 mg/dL is often
used to define parenteral nutrition-associated
cholestasis.
18
Progressively worsening cholestasis has
been associated with increased mortality. One report
associated a conjugated bilirubin level of 4 mg/dL for
at least 6 months after bowel resection with a 78% risk
of mortality.
19
In fact, parenteral nutrition-associated
liver
disease with subsequent liver failure is a leading
cause of death in patients with SBS.
20,21
In the absence
of irreversible liver damage, liver tests tend to return to
normal within 14 months after discontinuing
parenteral nutrition.
Several strategies can be employed to prevent or
treat parenteral nutrition-associated cholestasis. These
include early introduction and advancement of enteral
feeds, cycling and weaning parenteral nutrition, man-
agement of SBBO, and rapid and aggressive treatment
of sepsis. It is very important to initiate enteral feedings
whenever possible. Even small-volume trophic feeds can
improve intestinal motility, reduce bacterial transloca-
tion, and improve bile ow. Restriction of manganese
and copper intake in parenteral nutrition to avoid toxic
hepatic accumulation should be considered.
22
Intravenous lipid solutions have been implicated
in liver dysfunction. Currently available lipid solutions
in the United States are derived from soybean or
soybeansafower oil, and are rich in -6 fatty acids.
There is increasing evidence that substituting soybean-
based with sh oil-based lipid solutions (rich in -3
fatty acids) may reverse parenteral nutrition-associated
cholestasis.
23,24
Cycling parenteral nutrition (providing
the infusion over <24 hours/day) is thought to reduce
hepatic complications. When cycling is performed over
short periods, patients should be monitored for uctua-
tions in glucose levels. Advancing and tapering the infu-
sion over 1 or 2 hours may avoid such uctuations.
Ursodeoxycholic acid, a naturally occurring hydrophilic
bile acid, is often used to stimulate bile ow and displace
toxic bile acids. Ursodeoxycholic acid at 2030 mg/kg/
day can be provided in two or three divided doses. In
cases of advanced liver disease, intestinal or combined
intestinalliver transplantation may be the only life-
saving alternative.
Pruritis, which is associated with reduced bile
ow, can be a prominent symptom and difcult to treat.
Available options include soothing topical creams and
oral medications such as antihistamines, rifampin,
phenobarbital, cholestyramine, and ursodeoxycholic
acid. Further discussion on pruritus treatment can be
found in Chapter 7. Table 204 provides general recom-
mendations on the indications and dosing of commonly
used medications in SBS patients.
Bishop_Ch20_295-305.indd 301 4/6/10 4:14:50 PM
302 Section 3: Disorders of the Stomach and Intestine
D-Lactic Acidosis
D-Lactic acidosis occurs in some patients with SBS due
to colonic fermentation of undigested or partially
digested carbohydrates. Certain bacterial species, mostly
gram-positive anaerobes, such as lactobacillus and bi-
dobacterium, produce D-lactate that is subsequently
absorbed. In humans, D-lactate accumulation can occur
because of slow metabolism and clearance in addition
to excessive production. D-Lactic acidosis may clinically
manifest with recurrent episodes of weakness, clumsi-
ness, slurred speech, confusion, somnolence, unsteady
gait, or excessive irritability. The episodes are usually
episodic, lasting hours to days. This has been described
from a few months to many years after intestinal resec-
tion. The colon must be present for D-lactic acidosis to
occur. Normally D-lactate is undetectable in blood, so a
combination of a detectable D-lactate level, acidosis, and
abnormal neurologic symptoms is highly suggestive of
D-lactic acidosis. Treatment options can include a
restricted carbohydrate diet to minimize substrate deliv-
ery to the colon, and enteral antibiotics, such as neomy-
cin, kanamycin, or vancomycin.
26,27
Cholelithiasis
Following ileal resection, the enterohepatic bile salt circu-
lation is interrupted. Bile salt loss can exceed the livers
capacity to replenish losses, with a subsequent drop in the
bile salt concentration. This can alter bile composition
leading to increased saturation with cholesterol and sub-
sequent gallstone formation. Biliary stasis and sludge for-
mation, often associated with diminished oral/enteral
intake, can also be contributing factors (see Figure 202).
Renal Stones
Patients with SBS with ileal resection are at risk of devel-
oping renal stones. Ileal resection can result in increased
absorption of oxalate in the colon, which leads to hyper-
oxaluria and stone formation (see Figure 203). Such
patients should be referred to for evaluation and man-
agement by a specialist. Treatment includes adhering to
a low-oxalate diet, which excludes peanuts, pecans, tea,
cocoa, wheat germ, soybeans, and spinach. Other medi-
cal approaches include using cholestyramine to bind bile
salts and citrate to prevent further stone formation.
28
Surgical Intervention
Several surgical procedures can be utilized in the man-
agement of SBS. These include the establishment of
central venous access for delivery of parenteral nutrition,
feeding tube placement for enteral nutrition, creation of
ostomies, bowel-lengthening procedures, procedures to
delay intestinal transit, and transplantation.
Two intestinal-lengthening surgeries are currently
employed when dilated intestinal loops are present. The
Bianchi procedure involves dividing the dilated segment
longitudinally, creating two narrower segments that are
Indications and Dosing of Commonly Used Medications
Medication (Reference)
Total Daily Enteral
Dose (mg/kg/day)
Dosing Frequency
(Per Day) Indication Possible Side Effects
Famotidine
Proton pump inhibitor
Ursodeoxycholic acid
22
Cholestyramine
22
Rifampin
25
Metronidazole
0.51.2 (max
40 mg/day)
13
1530
240
10 (max 600 mg/day)
20 (max 750 mg/day)
2
12
3
3
2
34
GERD, gastric hypersecretion
GERD, gastric hypersecretion
Cholestasis, pruritus
Pruritus, diarrhea
Pruritus
SBBO
Constipation, diarrhea
Abdominal pain,
headache, diarrhea
Diarrhea, nausea,
vomiting
Constipation, nausea,
vomiting, abdominal
pain
Elevated liver tests,
nausea, loss of
appetite, urine
discoloration
Abdominal discomfort,
loss of appetite,
metallic taste, nausea
and vomiting
GERD 5 gastroesophageal reux disease; max 5 maximum dose; SBBO 5 small bowel bacterial overgrowth.
Table 204.
Bishop_Ch20_295-305.indd 302 4/6/10 4:14:50 PM
CHAPTER 20 Short Bowel Syndrome 303
anastomosed end-to-end (see Figure 204). The serial
transverse enteroplasty (STEP) procedure involves appli-
cation of a surgical stapler to the dilated loop at a right
angle on alternating sides to create a zigzag longer and
narrower intestinal segment (see Figure 205). The STEP
procedure is simpler and requires no anastomoses. Indica-
tions for undergoing intestinal-lengthening surgery
should include the presence of dilated intestinal loops and
dependence on parenteral nutrition without evidence for
further intestinal adaptation. Results from both lengthen-
ing procedures have been favorable by advancing enteral
nutrition, weaning parenteral nutrition and reversing its
complications, and avoiding transplantation.
29
Transplantation is the last surgical option for SBS
patients on prolonged parenteral nutrition who experi-
ence life-threatening complications such as liver failure.
Other cited indications for transplantation include fre-
quent episodes of severe dehydration despite parenteral
nutrition and intravenous hydration and recurrent cen-
tral line infections and thrombosis with progressive loss
of vascular access.
30
Types of transplants offered include
isolated intestinal, combined liverintestinal, and multi-
visceral transplantation. Isolated liver transplantation is
occasionally offered to a select population with liver
failure in whom good bowel adaptation and eventual
enteral autonomy are expected. Despite advances over
the last decade, intestinal transplantation is still associ-
ated with a high risk of infection, multiorgan failure,
rejection, and complications related to immunosup-
pression. The overall 3-year patient survival after intes-
tinal transplantation is about 50%.
3
Medical and other
surgical alternatives should therefore be maximally uti-
lized prior to considering transplantation.
CONCLUSION
SBS is a complex and life-threatening disease affecting chil-
dren with signicant intestinal loss. Management includes
meticulous nutritional support, with emphasis on early
advancement of enteral feeds, weaning from parenteral
nutrition, monitoring for complications, and addressing
possible associated liver dysfunction. Signicant liver dys-
function can lead to high morbidity and mortality in SBS
patients. The early involvement of an experienced pediat-
ric center can provide support from different subspecial-
ists. Intestinal transplantation may be needed when other
medical and surgical interventions have failed.
Ileal resection
Bile salt depletion
Fat malabsorption
Calcium soap formation
Reduced intraluminal
calcium (unavailable to
bind dietary oxalate)
Excessive oxalate
absorption
Renal oxalate stones
FIGURE 203 Oxalate stone formation.
Vitamin B12
malabsorption;
megaloblastic anemia
Ileal resection
Fat malabsortion
Renal
oxalate stones
Fat soluble
vitamin deficiencies
Failure to
thrive
Gallstones
Bile salt
malabsorption
FIGURE 202 Consequences of ileal resection.
Bishop_Ch20_295-305.indd 303 4/6/10 4:14:50 PM
304 Section 3: Disorders of the Stomach and Intestine
4. Gourevitch D. The anatomy and physiology of the small
bowel. In: Fielding JW, Hallissey MT, eds. Upper
Gastrointestinal Surgery. London: Springer; 2005:3944.
5. Vanderhoof JA, Young RJ. Enteral and parenteral
nutrition in the care of patients with short-bowel syn-
drome. Best Pract Res Clin Gastroenterol. 2003;17(6):
9971015.
6. Gupte GL, Beath SV, Kelly DA, Millar AJ, Booth IW.
Current issues in the management of intestinal failure.
Arch Dis Child. 2006;91(3):259264.
7. Duro D, Kamin D, Duggan C. Overview of pediatric short
bowel syndrome. J Pediatr Gastroenterol Nutr.
2008;47(suppl 1):S33S36.
Pre-lengthening Post-lengthening
FIGURE 205 Intestinal lengthening procedures: serial transverse enteroplasty
procedure.
A
C
B
D
FIGURE 204 Intestinal lengthening procedures: Bianchi procedure. (A) Split the mesentery; (B) divide
the bowel lengthwise; (C) separate one half from the upstream bowel; (D) make end-to-end anastomosis.
REFERENCES
1. Cole CR, Hansen NI, Higgins RD, et al. Very low birth
weight preterm infants with surgical short bowel syn-
drome: incidence, morbidity and mortality, and growth
outcomes at 18 to 22 months. Pediatrics. 2008;122(3):
e573e582.
2. Goulet O, Ruemmele F. Causes and management of
intestinal failure in children. Gastroenterology. 2006;130
(2 suppl 1):S16S28.
3. Goulet O, Ruemmele F, Lacaille F, Colomb V. Irreversible
intestinal failure. J Pediatr Gastroenterol Nutr.
2004;38(3):250269.
Bishop_Ch20_295-305.indd 304 4/6/10 4:14:50 PM
CHAPTER 20 Short Bowel Syndrome 305
8. Crenn P, Messing B, Cynober L. Citrulline as a biomarker
of intestinal failure due to enterocyte mass reduction. Clin
Nutr. 2008;27(3):328339.
9. Rhoads JM, Plunkett E, Galanko J, et al. Serum citrulline
levels correlate with enteral tolerance and bowel length in
infants with short bowel syndrome. J Pediatr.
2005;146(4):542547.
10. Wessel JJ, Kocoshis SA. Nutritional management of infants
with short bowel syndrome. Semin Perinatol.
2007;31(2):104111.
11. Cordano A. Clinical manifestations of nutritional copper
deficiency in infants and children. Am J Clin Nutr.
1998;67(5 suppl):1012S1016S.
12. Elliott TS. The prevention of central venous catheter-related
sepsis. J Chemother. 2001;13 Spec No 1(1):234238.
13. Onland W, Shin CE, Fustar S, Rushing T, Wong WY. Eth-
anol-lock technique for persistent bacteremia of long-term
intravascular devices in pediatric patients. Arch Pediatr
Adolesc Med. 2006;160(10):10491053.
14. Vanderhoof JA. New and emerging therapies for short
bowel syndrome in children. J Pediatr Gastroenterol Nutr.
2004;39(suppl 3):S769S771.
15. Ziegler MM. Short bowel syndrome in infancy: etiology
and management. Clin Perinatol. 1986;13(1):163173.
16. Jakubik LD, Colfer A, Grossman MB. Pediatric short
bowel syndrome: pathophysiology, nursing care, and
management issues. J Soc Pediatr Nurs. 2000;5(3):
111121.
17. Vanderhoof JA. Short bowel syndrome. Clin Perinatol.
1996;23(2):377386.
18. Drongowski RA, Coran AG. An analysis of factors con-
tributing to the development of total parenteral nutrition-
induced cholestasis. JPEN J Parenter Enteral Nutr.
1989;13(6):586589.
19. Teitelbaum DH, Drongowski R, Spivak D. Rapid develop-
ment of hyperbilirubinemia in infants with the short
bowel syndrome as a correlate to mortality: possible indi-
cation for early small bowel transplantation. Transplant
Proc. 1996;28(5):26992700.
20. Cooper A, Floyd TF, Ross AJ 3rd, Bishop HC, Templeton
JM Jr, Ziegler MM. Morbidity and mortality of short-bowel
syndrome acquired in infancy: an update. J Pediatr Surg.
1984;19(6):711718.
21. Simmons MG, Georgeson KE, Figueroa R, Mock DL. Liver
failure in parenteral nutrition-dependent children with
short bowel syndrome. Transplant Proc. 1996;28(5):2701.
22. Btaiche IF, Khalidi N. Parenteral nutrition-associated liver
complications in children. Pharmacotherapy.
2002;22(2):188211.
23. Gura KM, Lee S, Valim C, et al. Safety and efcacy of a
sh-oil-based fat emulsion in the treatment of parenteral
nutrition-associated liver disease. Pediatrics.
2008;121(3):e678e686.
24. Gura KM, Duggan CP, Collier SB, et al. Reversal of
parenteral nutrition-associated liver disease in two infants
with short bowel syndrome using parenteral sh oil:
implications for future management. Pediatrics.
2006;118(1):e197e201.
25. Yerushalmi B, Sokol RJ, Narkewicz MR, Smith D, Karrer
FM. Use of rifampin for severe pruritus in children with
chronic cholestasis. J Pediatr Gastroenterol Nutr.
1999;29(4):442447.
26. Zhang DL, Jiang ZW, Jiang J, Cao B, Li JS. D-Lactic acido-
sis secondary to short bowel syndrome. Postgrad Med J.
2003;79(928):110112.
27. Uribarri J, Oh MS, Carroll HJ. D-Lactic acidosis. A review
of clinical presentation, biochemical features, and patho-
physiologic mechanisms. Medicine (Baltimore).
1998;77(2):7382.
28. Morton AR, Iliescu EA, Wilson JW. Nephrology: 1. Inves-
tigation and treatment of recurrent kidney stones. CMAJ.
2002;166(2):213218.
29. Sudan D, Thompson J, Botha J, et al. Comparison of intes-
tinal lengthening procedures for patients with short bowel
syndrome. Ann Surg. 2007;246(4):593601.
30. Torres C. Assessment of intestinal failure patients.
In: Langnas A, Goulet O, Quigley E, Tappenden K, eds.
Intestinal Failure: Diagnosis, Management and Transplanta-
tion. 1st ed. Malden, MA: Wiley-Blackwell; 2008:117121.
Bishop_Ch20_295-305.indd 305 4/6/10 4:14:56 PM
You might also like
- Med Terminology in A Flash - CardioDocument66 pagesMed Terminology in A Flash - CardiobencleeseNo ratings yet
- Intestinal Failure and Short Bowel SyndromeDocument5 pagesIntestinal Failure and Short Bowel SyndromesivaNo ratings yet
- 55 Uses of Baking Soda For Health and HouseholdDocument99 pages55 Uses of Baking Soda For Health and HouseholdJennifer davis100% (1)
- Obstructive JaundiceDocument18 pagesObstructive JaundiceBellinda PaterasariNo ratings yet
- Antepartum Record Labor WatchDocument4 pagesAntepartum Record Labor WatchMaryJoy rosalesNo ratings yet
- Applied Biopharmaceutics & Pharmacokinetics, 5th EditionDocument1 pageApplied Biopharmaceutics & Pharmacokinetics, 5th Editionbencleese14% (7)
- Applied Biopharmaceutics & Pharmacokinetics, 5th EditionDocument1 pageApplied Biopharmaceutics & Pharmacokinetics, 5th Editionbencleese14% (7)
- Applied Biopharmaceutics & Pharmacokinetics, 5th EditionDocument1 pageApplied Biopharmaceutics & Pharmacokinetics, 5th Editionbencleese14% (7)
- Gallstones: Causes, Symptoms and Treatment of CholelithiasisDocument7 pagesGallstones: Causes, Symptoms and Treatment of Cholelithiasisjesabel_caraigNo ratings yet
- SURGERY Lecture 1 - Small Intestine (Dr. Mendoza)Document16 pagesSURGERY Lecture 1 - Small Intestine (Dr. Mendoza)Medisina101100% (1)
- Salbutamol Drug SummDocument1 pageSalbutamol Drug SummWarren100% (2)
- Chapter 5. Intravenous InfusionDocument22 pagesChapter 5. Intravenous InfusionbencleeseNo ratings yet
- Chapter 3. One-Compartment Open Model Intravenous Bolus AdministrationDocument23 pagesChapter 3. One-Compartment Open Model Intravenous Bolus AdministrationbencleeseNo ratings yet
- AABB Technical ManualDocument841 pagesAABB Technical ManualMarty DolceNo ratings yet
- Narrative Wellness Program 2021Document5 pagesNarrative Wellness Program 2021Chay Dayne100% (6)
- Diseases of GallbladderDocument85 pagesDiseases of GallbladderFatima Marwa Teo MaghinayNo ratings yet
- Gastric Outlet ObstructionDocument35 pagesGastric Outlet ObstructionsiddhantaNo ratings yet
- Beauty Care NC IIDocument84 pagesBeauty Care NC IIMairem BarrugaNo ratings yet
- Assessment Diagnosis Planning Intervention Rationale EvaluationDocument1 pageAssessment Diagnosis Planning Intervention Rationale Evaluationcamile buhanginNo ratings yet
- Leaky Gut Syndrome for Beginners - The Self-Help Book - How to Correctly Interpret the Symptoms of a Leaky Gut, Identify the Causes and Heal Your Gut Step by StepFrom EverandLeaky Gut Syndrome for Beginners - The Self-Help Book - How to Correctly Interpret the Symptoms of a Leaky Gut, Identify the Causes and Heal Your Gut Step by StepNo ratings yet
- Cholecystitis IntroductionDocument4 pagesCholecystitis IntroductionJechelle Ann Pabustan Martin-BoniquitNo ratings yet
- Case Scenario CholeDocument4 pagesCase Scenario CholeAlden MendozaNo ratings yet
- A Simple Guide to Short Bowel Syndrome, Diagnosis, Treatment and Related ConditionsFrom EverandA Simple Guide to Short Bowel Syndrome, Diagnosis, Treatment and Related ConditionsNo ratings yet
- Pathophysiology of DiarrheaDocument4 pagesPathophysiology of DiarrheaonewRAIN100% (1)
- Short Bowel SyndromeDocument50 pagesShort Bowel SyndromeAbdul QadirNo ratings yet
- PathophysiologyDocument8 pagesPathophysiologyadeNo ratings yet
- Removal of Intestine 1Document5 pagesRemoval of Intestine 1Eaint BoNo ratings yet
- Small BowelDocument7 pagesSmall BowelIfeanyichukwu OgbonnayaNo ratings yet
- Malabsorption Seminar: Causes and Nursing CareDocument42 pagesMalabsorption Seminar: Causes and Nursing CaresomivipinNo ratings yet
- Approach To The Patient With Gastrointestinal DiseaseDocument55 pagesApproach To The Patient With Gastrointestinal DiseaseHero StoreNo ratings yet
- Nutritional Support in Gastrointestinal Diseases: Compromised GutDocument18 pagesNutritional Support in Gastrointestinal Diseases: Compromised GutRoxana CristeaNo ratings yet
- Approach To The Gi DisorderDocument52 pagesApproach To The Gi Disordernathan asfahaNo ratings yet
- Crohn's Disease Malabsorption: Causes, Symptoms and Nursing CareDocument31 pagesCrohn's Disease Malabsorption: Causes, Symptoms and Nursing Caretatenda chitindiNo ratings yet
- Diarrea Crónica UPTODATEDocument6 pagesDiarrea Crónica UPTODATEConsuelo RiveraNo ratings yet
- PERSISTENT DIARRHEA IN NEONATES AND INFANTS UNDER 6 MONTHSDocument6 pagesPERSISTENT DIARRHEA IN NEONATES AND INFANTS UNDER 6 MONTHSConsuelo RiveraNo ratings yet
- Short Bowel Syndrome-Sindrom Usus PendekDocument42 pagesShort Bowel Syndrome-Sindrom Usus PendekAry RachmantoNo ratings yet
- Motility of SMALL INTESTINE, Malabsorbtion and DiarrhoeaDocument32 pagesMotility of SMALL INTESTINE, Malabsorbtion and DiarrhoeaWallerian DegenrationNo ratings yet
- Physiology, Large Intestine - StatPearls - NCBI Bookshelf PDFDocument3 pagesPhysiology, Large Intestine - StatPearls - NCBI Bookshelf PDFMichael MengNo ratings yet
- Nutrients: Nutritional Feeding Strategies in Pediatric Intestinal FailureDocument14 pagesNutrients: Nutritional Feeding Strategies in Pediatric Intestinal FailureKatherin AmadoNo ratings yet
- Management of Short Bowel Syndrome (SBS) and Intestinal FailureDocument9 pagesManagement of Short Bowel Syndrome (SBS) and Intestinal FailureIsabella María GantivarNo ratings yet
- Managing The Adult Patient With Short Bowel SyndromeDocument18 pagesManaging The Adult Patient With Short Bowel SyndromeGar BettaNo ratings yet
- Seow-Choen F. Surgery For Haemorrhoids: Ablation or Correction. Asian J Surg 2002 25: 265-266Document2 pagesSeow-Choen F. Surgery For Haemorrhoids: Ablation or Correction. Asian J Surg 2002 25: 265-266l10n_assNo ratings yet
- Anatomy and functions of the digestive tractDocument11 pagesAnatomy and functions of the digestive tractrahtu suzi ameliaNo ratings yet
- GallstoneDocument6 pagesGallstoneMushfique HussainNo ratings yet
- Melena, Diarrhea, NauseaDocument5 pagesMelena, Diarrhea, Nauseakelompok 6B IDIKNo ratings yet
- Chronic Diarrhea GuideDocument14 pagesChronic Diarrhea GuideDini NanamiNo ratings yet
- Osmotic Diarrhoea: Difficile)Document4 pagesOsmotic Diarrhoea: Difficile)Marwan M.No ratings yet
- Intestine CleanseDocument12 pagesIntestine CleansemmyemailNo ratings yet
- Digestive and Liver DiseaseDocument9 pagesDigestive and Liver DiseasemattNo ratings yet
- Case Stydy On CholithiasisDocument29 pagesCase Stydy On CholithiasisDaniel TalleyNo ratings yet
- Shortbowelsyndrome 170109125846Document40 pagesShortbowelsyndrome 170109125846noviaayularasatiNo ratings yet
- მსხვილი ნაწლავი 1Document36 pagesმსხვილი ნაწლავი 1Malak A MahadeenNo ratings yet
- Gastric Bypass SurgeryDocument16 pagesGastric Bypass SurgeryAhmad Abu TahaNo ratings yet
- Physiological Functiond of The Colon - CompressedDocument25 pagesPhysiological Functiond of The Colon - CompressedmohamedhazemelfollNo ratings yet
- 1malabsorption SyndromeDocument6 pages1malabsorption SyndromeDranreb Berylle MasangkayNo ratings yet
- Disorders of The GallbladderDocument67 pagesDisorders of The GallbladderGeofry OdhiamboNo ratings yet
- PDF Document 5Document28 pagesPDF Document 5Kirstin del CarmenNo ratings yet
- physiology8weekDocument16 pagesphysiology8weekAnshulNo ratings yet
- Jaundice Revisited: Recent Advances in The Diagnosis and Treatment of Inherited Cholestatic Liver DiseasesDocument13 pagesJaundice Revisited: Recent Advances in The Diagnosis and Treatment of Inherited Cholestatic Liver Diseasesmiss betawiNo ratings yet
- Bases Da Fisiopatologia Da Diarreia (The Pathophysiology of Diarrhea)Document18 pagesBases Da Fisiopatologia Da Diarreia (The Pathophysiology of Diarrhea)MujiartiNo ratings yet
- sibo sunumDocument37 pagessibo sunumSeda KaraçamNo ratings yet
- Short Bowel Syndrome: Epidemiology and Etiology: Paul W. Wales, MD, Emily R. Christison-Lagay, MDDocument7 pagesShort Bowel Syndrome: Epidemiology and Etiology: Paul W. Wales, MD, Emily R. Christison-Lagay, MDSubha ManivannanNo ratings yet
- Malabsorption: Exocrine Pancreatic InsufficiencyDocument6 pagesMalabsorption: Exocrine Pancreatic InsufficiencyDranreb Berylle MasangkayNo ratings yet
- Equine Colic (Adult Horses) : EtiologyDocument22 pagesEquine Colic (Adult Horses) : EtiologyroonyNo ratings yet
- PathoPhysiology of Diarrhea-PattyDocument5 pagesPathoPhysiology of Diarrhea-Pattyjap kurotsukiNo ratings yet
- Pathophysiology of DiarrheaDocument3 pagesPathophysiology of DiarrheaFathur RahmatNo ratings yet
- Norovirus RotavirusDocument4 pagesNorovirus RotavirusShiella Heart MalanaNo ratings yet
- Intestinal Malabsorption in The Elderly: Peter R. HoltDocument7 pagesIntestinal Malabsorption in The Elderly: Peter R. HoltIqbal Z AssyidiqieNo ratings yet
- Malabsorption SyndromesDocument14 pagesMalabsorption SyndromesRAFI ABRAR PRATAMANo ratings yet
- Diarrhea Cmap ScriptDocument5 pagesDiarrhea Cmap ScriptmaryNo ratings yet
- The #1 Danger Of Prolonged Fasting You Have To Know About - Based On The Teachings Of Dr. Eric Berg: The Most Important Risk To UnderstandFrom EverandThe #1 Danger Of Prolonged Fasting You Have To Know About - Based On The Teachings Of Dr. Eric Berg: The Most Important Risk To UnderstandNo ratings yet
- Constipation: How To Treat Constipation: How To Prevent Constipation: Along With Nutrition, Diet, And Exercise For ConstipationFrom EverandConstipation: How To Treat Constipation: How To Prevent Constipation: Along With Nutrition, Diet, And Exercise For ConstipationNo ratings yet
- Existing Efforts Connecting The CountryDocument70 pagesExisting Efforts Connecting The CountrybencleeseNo ratings yet
- Carbohydrates and SweetnersCDDocument30 pagesCarbohydrates and SweetnersCDbencleeseNo ratings yet
- Fat and SodiumcdDocument20 pagesFat and SodiumcdbencleeseNo ratings yet
- Newsletter Spotlight Spring 2009Document16 pagesNewsletter Spotlight Spring 2009bencleeseNo ratings yet
- NewesletterSpotlight Winter 2009Document16 pagesNewesletterSpotlight Winter 2009bencleeseNo ratings yet
- Test Taking Tips Sample ChapterDocument51 pagesTest Taking Tips Sample ChapterbencleeseNo ratings yet
- Newsletter Spotlight Spring 2009Document16 pagesNewsletter Spotlight Spring 2009bencleeseNo ratings yet
- Vitamins Minerals FibercdDocument15 pagesVitamins Minerals FibercdbencleeseNo ratings yet
- Nwewsletter Spotlight Fall 2008Document16 pagesNwewsletter Spotlight Fall 2008bencleeseNo ratings yet
- Chapter 7. Pharmacokinetics of Oral AbsorptionDocument28 pagesChapter 7. Pharmacokinetics of Oral AbsorptionbencleeseNo ratings yet
- Test Taking Tips Sample ChapterDocument51 pagesTest Taking Tips Sample ChapterbencleeseNo ratings yet
- Harrison's Online ContentsDocument18 pagesHarrison's Online Contentsbencleese0% (1)
- The Practice of Medicine - CH 02Document22 pagesThe Practice of Medicine - CH 02bencleeseNo ratings yet
- The Practice of Medicine - CH 01Document15 pagesThe Practice of Medicine - CH 01bencleeseNo ratings yet
- Summary of - House Bill 98-Senate Bill 99, Diabetes CoverageDocument2 pagesSummary of - House Bill 98-Senate Bill 99, Diabetes CoveragebencleeseNo ratings yet
- Chapter 1. Introduction To Medical Research - SectionsDocument1 pageChapter 1. Introduction To Medical Research - SectionsbencleeseNo ratings yet
- Basic & Clinical Biostatistics, 4th EditionDocument6 pagesBasic & Clinical Biostatistics, 4th EditionbencleeseNo ratings yet
- Chapter 1. Introduction To Medical ResearchDocument10 pagesChapter 1. Introduction To Medical ResearchbencleeseNo ratings yet
- Pay-For-Performance Medicine-Quality or QuagmireDocument3 pagesPay-For-Performance Medicine-Quality or QuagmirebencleeseNo ratings yet
- LEAF 2012 Owner ManualDocument354 pagesLEAF 2012 Owner ManualbencleeseNo ratings yet
- Basic & Clinical Biostatistics, 4th Edition: SearchDocument1 pageBasic & Clinical Biostatistics, 4th Edition: SearchbencleeseNo ratings yet
- LEAF 2012 Quick Reference GuideDocument24 pagesLEAF 2012 Quick Reference GuidebencleeseNo ratings yet
- LEAF 2012 Quick Reference GuideDocument24 pagesLEAF 2012 Quick Reference GuidebencleeseNo ratings yet
- LEAF 2012 Owner ManualDocument354 pagesLEAF 2012 Owner ManualbencleeseNo ratings yet
- NIH CancerDocument198 pagesNIH CancerAnonymous KgUtPlkjNo ratings yet
- FAQ MaV MaYojanaDocument3 pagesFAQ MaV MaYojanaDhruv GhevariyaNo ratings yet
- A Study of Comparative Efficacy of Baclofen Vs AcamprosateDocument6 pagesA Study of Comparative Efficacy of Baclofen Vs Acamprosatebaclofen-research100% (1)
- Ss811e MSDS enDocument11 pagesSs811e MSDS enJeric SalcedoNo ratings yet
- Alprazolam PoisoningDocument4 pagesAlprazolam PoisoningRahmat AkmalNo ratings yet
- Sign 110Document84 pagesSign 110speedo78No ratings yet
- Eie No.1 - Q3Document4 pagesEie No.1 - Q3Ma'am Janika Janek MetierreNo ratings yet
- Uganda Universities and Courses For Student Loan SupportDocument5 pagesUganda Universities and Courses For Student Loan SupportThe Campus TimesNo ratings yet
- Tsca Toxic Substanteces Control ActDocument1 pageTsca Toxic Substanteces Control ActAlbert Casanova FernandezNo ratings yet
- Home Remedies For UtiDocument5 pagesHome Remedies For UtiShiro KiryuuNo ratings yet
- When A Child Dies LeafletDocument2 pagesWhen A Child Dies LeafletCruseBexleyNo ratings yet
- DWATCHDocument92 pagesDWATCHFawaz SayedNo ratings yet
- EmgnhDocument321 pagesEmgnhJess GoNo ratings yet
- HypotrophyDocument3 pagesHypotrophyopeyemi daramolaNo ratings yet
- User Manual 2 3875358Document23 pagesUser Manual 2 3875358Maria Camila RestrepoNo ratings yet
- Pediculosis: Pubis (Pubic Louse)Document4 pagesPediculosis: Pubis (Pubic Louse)christian quiaoitNo ratings yet
- Abbreviated New Drug ApplicationDocument3 pagesAbbreviated New Drug Applicationlalit_draNo ratings yet
- Nursing Care For Patients With Endocrine DisordersDocument4 pagesNursing Care For Patients With Endocrine DisordersMark Russel Sean LealNo ratings yet
- CHAPTER 2 - Ethics in Pharmacy PracticeDocument15 pagesCHAPTER 2 - Ethics in Pharmacy PracticeMona GhazyNo ratings yet
- Effects of Implementation of Focus-Pdca Model OnDocument14 pagesEffects of Implementation of Focus-Pdca Model OnSofiyullohNo ratings yet
- Jurnal Tortikolis Hanif Dan AdeDocument14 pagesJurnal Tortikolis Hanif Dan AdeIlham Amal MNo ratings yet
- Research in Social and Administrative PharmacyDocument4 pagesResearch in Social and Administrative PharmacyAndrea MendozaNo ratings yet
- Comorbid Drug Use Disorders and Eating Disorders - A Review of Prevalence StudiesDocument12 pagesComorbid Drug Use Disorders and Eating Disorders - A Review of Prevalence StudiesJhon QuinteroNo ratings yet