Professional Documents
Culture Documents
Mapa Metabólico
Uploaded by
nelsonfqCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mapa Metabólico
Uploaded by
nelsonfqCopyright:
Available Formats
C ONH 2 C OO
HY A L UR ONIC A C ID DE R MA T A N B L OOD G R OUP A L G INA T E S O-A NT IG E NS S TAR CH G LY C OG E N + O O C OO
P G LY C OP R OT E INS S UB S T A NC E S C H 2 C OO OH
+
N
R ibos e
O O
Adenos ine(P )
N
R ibos e -O-P -O-P -O -Adenos ine + NIC OT INA T E C OO C OO
O G A NG L IOS IDE S P E P T IDO- C H 2 OH C H 2 OH
-O - P - O - P - O- N
11 + C OO
MUC INS G LY C A N C H C HIT IN C HONDR OIT IN P E C T IN INUL IN C E L L UL OS E O O NH NH O O O O R ibos e- P 2. 4. 2. N N RP
L 2 OH HO Indoleac etate Indoxyl + 6.3.5.1 2.7.7.18
Quinolinate- A
O
O O O (A uxin) NA D( P ) 6.3.1.5 Des amino-NA D Nic otinate- 2.4.2.19
Y C HOH
O
C OO - 6.3.2.7-10
C OO - 2.4.1.68
2.4.1.69
1.1.1.132 HO C H 3
OH OH OH
1.2.3.7
nuc leotide nuc leotide R
AcNH 2.4.2.19
S C HOH
C H 2 OH 2.4.99.7 6.3.2.13 HO O OP P U HO OH OP P U C H3
O OP P T
2.4.1.29
OH OH
HO
+
C H 2 C H(NH 3)C OO HO C H 2 C H 2 NH 2 HO C H 2 C H 2 NHC OC H 3 C H 3O C H 2 C H 2 NHC OC H 3 C OO O
OP C 2.4.1.17 L A C TOS E
A C H 3C H NHAC
UDP -
OH
HO HO OP P G
T DP -R hamnos e
OH OH
C H 2OH
C H 2 C HO
NH NH NH NH N
C OO
M
C HO C OO- G DP - 2.4.1.21 O NH NH
5-Hydroxy- 4.1.1.28
5-Hydroxytryptamine 2.3.1.5
N-A c etyl-s erotonin 2.1.1.4
N-A c etyl-5-O-methyl-s erotonin Quinolinate A
UDP -N-A c -Muramate Iduronate OH
Mannuronate C H3
2.4.1.1
etc.
2.4.1.11 HO
2.4.1.21 3.2.1.23 Indole- Indole (S E R OTONIN) (ME L A TONIN)
C G DP -F uc os e O tryptophan T
C MP -N-A c etyl OH OH 2.7.1.38 ac etaldehyde 1.14.16.4
H neuraminate
HO
C H 2 OH
O C OO 5.1.3.12
C H 2 OH
O
5.1.3.13 O
OH OP P T OH 4.1.1.43 +
+
C OO
I
+ C O C H 2 C H(NH 3 )C OO C OO
A C HOH
C HOH
O
C OO
2.4.1.16
C OO -
4.2.1.47 2.4.1.33
OH A DP - C H 2OH G A L A C TOS E
C H 2 C OC OO
C O C H 2 C H(NH 3 )C OO
C HO
C O C H 2 C H(NH 3 )C OO
NH 2 NH 2 OC H OC H NH 2
C
AcNH NH 2
R C H 2 OH OH OP P U O
HO OH HO OP P G C H 2 OH
O
T DP -4-Oxo- G luc os e
O
C H 2 OH 4.1.99.1 NH
3.5.1.9
NH 2 OH OH C OO C OO
K ynurenine 1.14.13.9 3-Hydroxy
OH
OH 2.7.7.43 HO 6-deoxygluc os e 2.7.1.6
NH
F ormylkynurenine 3.7.1.3
3-Hydroxy 1.13.11.6 2-A mino-3-c arboxy 4.1.1.45
2-A minomuc onate-
I 3.1.3.29
1.1.1.158 C H 2 OH
O
NHC OC H 3 G DP -Mannos e HO OH OP P U HO
O
Indolepyruvate kynurenine anthranilate muc onate s emialdehyde 6-s emialdehyde A
OH OP P U
D HO UDP -N-A c - C H 2 OH
HO OH HO OH
G DP -G luc os e HO OH OP 2.4.1.22 1.13.11.11 C OO H H C atec hol M
N-A c -Neuraminate G alac tos amine OH
UDP -G luc os e
E
1.
HO O
UDP -
O
MA NNOS E + C OO
I
4.
(S ialate) OP P U 2.7.7.13 4.2.1.46 OH C H 2 C H(NH 3 )C OO NH C C C H C H 2O P 1.2.1.32
1.
C -C H(OH)C H(OH)C H 2 O P
S
C H2 C C OO
1. 1. 1. 22 G alac tos e-P C H 2 C H 2 NH 2 HOC -C H(OH)C H(OH)C H 2 OP C OO
19
C H 2O P
C OO
NHAC
O G alac turonate 2.7.1.7 2.7.7.27 NH
CH CH OH OH NH 2
1.14.12.1 N
O HO OH HO O P C H 2 OH NH 4.2.1.20 N N O
H H NH 2
UDP -N-A c -
3.1.3.29 5.1.3.7 Mannos e-1-P
T DP -G luc os e
2.7.7.34 2.7.7.9 HO
O
T ryptamine
4.1.1.28
T R Y P TOP HA N Indole-3-glyc erol-P 1-(o-C arboxy phenylamino)
4.1.1.48
N-(5-P -R ibos yl)
2.4.2.18
A nthranilate
OOC
OOC O
AC NH
HO OH G luc os amine
4.1.3.20 HO OH OP P U 5.1.3.6 C H 2O P
O
5.1.3.2
C OO 1-deoxyribulos e-5-P anthranilate 2-A mino
OH 2.7.7.10
pyruvate OH 2.7.7.12 OH OP P U OC
HO
muc onate
N-A c -Mannos amine-6-P UDP -G luc uronate 5.4.2.8
HO OH HO OH
2.7.7.24 C H 2 OH
O OH P OC H 2 C H 2
C OO C OO C OO
C OO C OO C OO
4.1.3.27
OOC C H 2 C OC OO
A
C H 2 OH
C H 2 OH
O
C H 2 OH
O 4.1.3.20 O
C H 2O P
O
HOC H HC OH
C OH OH HO OH
C H2 C H2
C
OH O-C -C OO
H 2.7.1.60 Mannos e-6-P 2.4.1.13 C H 2O P HO OH OP UDP -G alac tos e H OH
4.6.1.3
O OH
4.2.1.10
O
OH
1.1.1.25
OH
2.7.1.71
P O
OH
2.5.1.19
P O
OH OH OC -C OO
5.4.99.5
OH I
S hikimate-5 4.6.1.4
AC NH
HO OH OH HO OH OP P U HO OH OP HO OH O HO 3-Deoxy-D-arabino- Dehydro- Dehydro- S hikimate S hikimate-3-P C horis mate P rephenate D
E OH 2.4.1.9
heptulos onate-7-P quinate s hikimate PEP
enolpyruvate 3-P
X
NHAC NHC OC H 3 5.4.2.3
N-A c -G luc os amine-6-P
NHC OC H 3 G luc os e-1-P O O
OH C H 2 C OC OO
+
C H 2 C H (NH 3 ) C OO C H 2 C H(NH 3 ) C OO
+
S
N-A c -Mannos amine UDP -N-A c -G luc os amine N-A c -G luc os amine-1-P HO OH OH C H 2 OH
O C H 2 C OO
C H 2 C OC OO
4.2.1.51
2. 3. 1.
O 5.1.3.14
C OO
2.7.7.23
OH OH OH H H OH
4 NH 2
-OOC
O
C H 2 C OO
O
C H 2 C OO
1.3.1.13
S OH
5.3.1.8 G luc os amine-6-P HO OH OH
C OO OH OH OH
F umaryl 5.2.1.2 Maleyl 1.13.11.5 Homogentis ate
O C C C CO C H 2 OH 1.13.11.27 2.6.1.5 1.14.16.1 2.6.1.5
OH HOC H 2 Hydroxyphenyl T Y R OS INE P HE NY L A L A NINE P henylpyruvate
E OH H OH OH OP
OH OH H OH
ac etoac etate ac etoac etate pyruvate 4. 3. 1.
5
C OO -
1.3.1.13 1.3.1.13
S HOC H 2 C C C C HO OH OH HO OH HO OH
OH
F ruc tos e S UC R OS E 3.2.1.26 3.2.1.48
G L UC OS E C HOHC H 2 NHC H 3 C HOHC H 2 NH 2 C H 2 C H 2 NH 2
+
C H 2 C H (NH 3 ) C OO-
H OH H H OH OH 4 .1
G ulonate
1.1.1.19
G luc uronate
1.13.99.1
Inos itol
3.1.3.25
Inos itol-P 5.5.1.4
.1 .2
5
C H 2 C H 2 NH 2 C H=C HC OO C
OH H OH OH OH H OH 5.4.2.2 OH OH OH OH Ubiquinone C innamate A
OH H OH OH 1. 1. 1.
21
ATP OH OH OH OH
1.14.16.2
Menaquinone
3.1.1.18 HOC H 2 C
H
C C
H
C
H
CO HOC H 2 C
H
C C
H
CO CO
HOC H 2 C C C C CO 1.1.1.14 H H OH H C H 2O P
O
3.1.3.9 2.7.1.2 E pinephrine 2.1.1.28
Norepinephrine 1.14.17.1
Dopamine 4.1.1.28
Dopa
+
C H 2 C H (NH 3 ) C OO
OH
T yramine 1.14.13.11 T
2.7.1.1
O O H O HOC H 2 C C C C C H 2 OH
2.6.1.16 (A drenaline)
C HOHC H 2 NH 2
(Noradrenaline)
HN O E
G ulonolac tone 1.1.3.8 2-Oxogulonolac tone A S C OR B A T E OH OH H OH O A DP .1 .6
1.10.2.1
S orbitol
2. 7 HO OH C H 2 OP 2 .1
1.14.18.1 H2 I I C H=C HC OO
C oumarate
C
1.1.1.45
OH H OH OH H OH H
1.10.3.3 . 1. 1.1.1.49 α-T oc opherol O C
O
HOC H 2 C C CO C C OO - C O C OO -
4 OH
O
C H(OH)C OO OH
OC H 3
C HOHC H 2 OH C H-C OO O
H
H OH H
HOC H 2 C
H
C
OH
CO HOC H 2 C
H
C CO
O
CO CO
2.7.1.3 P -G luc ono G luc os e-6-P Normetepinephrine
(V itamin E )
O
+
NH 3 O
OH
O
1.1.1.130 .1 7
P 3-Dehydrogulonate 2, 3-Dioxogulonate Dehydroas c orbate .1 lac tone HO OH OH OC H 3 (Normetadrenaline) OC H 3 Dopaquinone I I
E
H H OH H
C C OO -
3 .1
NADP + OH OH
1 .4
.3 .4 OH O NH OH L
P OC H 2 C C C 1.14.18.1
N
OH H OH H H OH H H
5.3.1.8 5.3.1.9 4-OH-3-Methoxy- 4-OH-3-Methoxy- P las toquinone ME L A NIN T HY R OXINE L IG NIN T annins P lant P igments A
6-P -G luc onate NA DP H
OH OH H OH
HOC H 2 C C C C HO HOC H 2 C C C C HO HOC H 2 C C C O C H 2 OH
H H OH
D-mandelate phenylglyc ol M
T H OH H OH OH H OH OH
O L -Xylos e 4.1.1.34 D-A rabinos e 5.3.1.3
D-R ibulos e 2.7.1.47 HOC H 2 C C C CO C H 2O P NADP +
H H HO
C H 2O P
O NHC OC H 2 NH 2 NH N
OOC -C H-C H 2 C OO
O
I
NH OOC
S OH H H OH H OH H
OH OH H
F ruc tos e-1-P
1.1.1.44
2.2.1.1 P OC H 2 C C C C O C H 2 OH H 2C C HO H 2C C HO
HC
N
CH
C
CH
HNC O C N
CH H 2N
C
C
N
CH
N
E HOC H 2 C C C C H 2 OH HOC H 2 C C CO C H 2 OH P OC H 2 C C CO C H 2 OH
H H H NA DP H
OH OH H
OH OH
OC
NH
RP
HN C
NH
RP
H 2N C
N
RP H2N
C
N
RP
H 2N C
N
RP H 2N
C N RP E
H OH OH H OH
2.7.1.53
H OH 5.1.3.1 F ruc tos e-6-P G lyc inamide- 2.1.2.2 F ormyl 6.3.5.3 F ormyl 6.3.3.1 5-A mino 4.1.1.21 5-A mino-4-imidazole 6.3.2.6 5-A mino-4-imidazole 4.3.2.2 5-A minoimidazole S
S L -A rabitol L -Xylulos e L -Xylulos e-5-P HOC H 2 C C C C H 2 OH H H
ribos yl-P glyc inamide-R P glyc inamidine-R P imidazole-R P c arboxylate-R P (N-s uc c inylc arboxamide)-R P c arboxamide-R P
O
C C N
OH OH H H OH H H OH H OH OH OH
R ibitol
P OC H 2 C C C O C H 2 OH ATP H2N
HC O C
CH
HOC H 2 C C C C HO HOC H 2 C C C C H 2 OH HOC H 2 C C C C HO OH OH H H 2.7.1.11 NH
O
NH O
2.1.2.3 NH N RP
H 2 NC ONH 2 H 2N C OO NH 2 H2N OC C N O N
H H OH 1.1.1.10 OH H OH OH H OH D-R ibulos e-5-P P OC H 2 C C C HO 3.1.3.11 CO HN C CO HN
C
C
C
Inos ine F ormylamido-
5 .3 2.7.1.47 A DP 6.3.4.13 Urea OC C
CO
OC C OC C CH HN C CH 3. 2. 2.
2 3. 1. 3.
5 imidazole-
L -A rabinos e .1 .4 Xylitol D-Xylos e H H H OH OH
N H NH N H NH N NH OC C HC C
OH H H OH OH OH OH HOC H 2 C C C C HO 5.1.3.1
E rythros e-4-P 3.5.3.4 H
3.5.2.5
H
1.7.3.3
H
1.1.1.204
NH
1.1.3.22
NH N NH
2.4.2.1 c arboxamide-R P
OH OH OH
H H HO
+ A llantoate A llantoin UR A T E 1.1.3.22 Xanthine Hypoxanthine 3.5.4.10 O
HOC H 2 C C C C HO HOC H 2 C C C O C H 2 OH P OC H 2 C C CO C H 2 OH P OC H 2 C C C C O C H 2O P C H 2 (NH 3 )C OOH NH 2 C N
N
D-R ibos e .1 .4
NH 2
N N HN C
H OH OH H H
L -R ibulos e 2.7.1.16 L -R ibulos e-5-P
H H
5.1 .3. 4 5.3.1.6
H OH OH OH H
2 .6 .1 0 G LY C INE N CH O O O HC
CH NH 2 N
C
OOC -C H-C H 2 C OO
HC C
CH
P
L -L yxos e C C C O C H 2 OH .1 20 N N NC N N RP
H OH 2 .7
P OC H 2 4.1.2.-
2.2.1.2 F ruc tos e- C H 2O P
O NH 2 1 .4
1.
1.
O HC
N N -O P ~O P ~O P O C H
2 O HC C
CH NH
C N
INOS INE -P U
1 .1 .1 OH H
2. O P O C H2 O N R P (P ) F umarate A s partate
.9 HOC H 2 C C CO C H 2 OH
.1 .1
5 D-Xylulos e-5-P 1: 6-bis -P OOC C H 2 NHC H 3
O O O N N
HC
C
C
CH (IMP ) R
S arc os ine OH OH 2.7.4.3
OH H
D-Xylulos e
2.7.1 .17 OH OH
G lyoxylate
O
OH
4.6 .1. 1 ATP 2.7.4.6
A DP
2.7.4.4 4.3.2.2
N NH R P
A denylo- 6.3.4.4 O
C N
I
P -R ibos yl 1.5.99.2 A DE NOS INE -P N
C yc lic A MP (A MP ) s uc c inate 3.1.4.6
HN C CH
H+
H H H amine 1.4.4.2 1.17.4.1 1.1.1.205 OC C
N RP E
H+ PHOTO- H+ P OC H 2 C C C C HO
H H H OH
OOC C H 2 N(C H 3 ) 2 d-A DP A denine 2.4.2.1
N
H
H+
2H+ yclic Ph
otophosphoryla SYSTEM
tion l
c
n-cycli electr
P OC H 2 C C C C C O C H 2O P 4.1.2.13 Dimethylglyc ine 2.7.4.6 3. 5. 4.
XA NT HOS INE -P S
No (electric curre on fl
OH OH OH 2.7.7.7 2.4.2.15
PHOTO-
SYSTEM C
2H+ H+ nt) o D-R ibos e-5-P S edoheptulos e-P P
OH OH OH H
2.1.1.5 2 .7 .7
.7 DNA 2. 7. 7. 7
d-G T P d-G DP 3 (XMP )
O
II
Ferredoxin 1 .1 G uanine 6.3.4.1 C N
w
H+ 7 .4 2. 4. 2.
P *- 2.2.1.1 OOC C H 2 N(C H 3 ) 3
+
d-A T P 2 .7
.7 .7 d-C T P .1 1 6.3.5.2
HN
H 2N C
C CH
H 2e- PQ 2e-
2e H+ C
PQ
_
. P OC H 2C HOHC HO
B etaine F OL IC ATP 2.7.7.6
R NA 2.7.7.6
GTP 2.7.4.6
G DP 2.7.4.8
N N RP
O QB
PQH2 1e- 1e-
PQ
H+ 3-P -G lyc eraldehyde 1.2.1.8 2.1.2.1 A C ID 2 .7
T T P 2.7.4 1. 17 .4 G UA NOS INE -P
Fe-S NADP+ HOC H 2 C OC H 2 OP .7 .6
T PQH2 Cyt bf
Dihydroxy-
2.2.1.1
.1 + C1 .6
.1 (G MP )
5 .3 .1
QA 2.4.2.14 OHC C H 2 N(C H 3 ) 3
O 2e- A1
H+
H+
ac etone-P Pi 6.3.4.7 B etaine P OOL NH 2
P
Pi 20 O 2 .7 T DP
O O C
S Pheophytin Cyt bc Chl.A0 (G lyc erone-P ) 5 .3 .1
.1 aldehyde 4.1.2.5 4. 2. 1. C O
.7 .6 C C N CH
Y
Y 2PQ .
_ 2e-
2PQ NADPH+H+ NA D+
C H3
C H3
HN C H-C H 3
HN
C
C C H3
2 .7
.4 .9 HN
C -C H 3
CH 2.1.1.45
HN CH
OC CH
P680
Chl.a 2PQH2 P700 Pi ADP
2.7.1.28 1.1.99.1 H 2 NC ONHC H 2 C HC OO
OC CH2
OC CH
OC
N OC CH
N DP
N
DP R
N 2e-
2e-
Fe-S 2e- Cyt.f
PC
H+
Glyceraldehyde
+
HOC H 2 C H 2 N (C H 3) 3
H 2 NC H 2 C HC OO
3-A mino- ß-Ureido
NH
Dihydro
NH
T hymine
2.4.2.4
DP
d-UMP
3.5.4.12
d-C MP
2.7.4.14
d-C DP I
T Mn H+
H+
1.2.1.12 C HOL INE 3.5.1.6 3.5.2.2 1.3.1.2 T HY MIDINE -P
PC PC
Ribulose-1,5-bis-P is obutyrate is obutyrate thymine O
M
α
α α
1.2.1.13 + O 2 .4 .2 NH 2
Pi
1
H 4H + HOC H 2 C H(NH 3 )C OO 2. 1 C C C
a
.4
NA DH 4.2.1.22 . 1. HN C H2 HN CH
8
I
β
H+ H+ H+ A DP N CH 1.17.4.1
S E R INE
8
H 2O Translocated protons
2
13
E
β
H+ C H2
CO2 OC OC CH CH
1
2.6.1.51 H 2 NC ONHC H 2 C H 2 C OO OC
H+ H+ 4.1.1.11
S H+
H+ H+ H+ H+ H+ H+ C H 2O P H 2 NC H 2 C H 2 C OO NH N H NH D
3
3
H+ γ O
P OC H 2C HOHC OO P 1.4.1.7 3.5.1.6 C arbamoyl 3.5.2.2 Dihydro- 1.3.1.2 3.5.4.1 C DP
ATP Fixation ß-A lanine Urac il C ytos ine
I O2 Protons from Water H+ H+ H+
1: 3-bis -P -G lyc erate ß-alanine urac il NH 2
I
H+
εε
2
H+
c
2.7.6.1
2
H+ H+ H+ β2 HOC H 2 C OC OO -OOC O O C
O
H+ C H 2.7.4.6
α
H+ H+ H+ H+ H+ O
α
OP OP 3.1.3.3 O C
S H+ H+
Hydroxy- NH
2 C H2
C C HN
C
CH C HN CH N
N
3
THYLAKOID LUMEN
OH OH C H2 CH HN CH CH
α
HN OC
ATP ATP HN 2.4.2.9
H+
THYLAKOID MEMBRANE P -R ibos yl-P P pyruvate OC
N
C H-C OO
OC C H-C OO OC C -C OO OC C -C OO OC N C H
OC
N
CH N
E
β
+ N R PPP RPPP
ATP synthase A DP P OC H 2 C H(NH 3 )C OO H NH NH RP RP 6.3.4.2
A DP C arbamoyl Dihydro Orotate Orotidine-P Uridine-P UDP UR IDINE - C Y T IDINE - S
STROMA 3.6.1.34 P hos pho- 3.5.2.3 4.1.1.23 (UMP ) 2.7.4.4
CHLOROPLAST OUTER MEMBRANE 2.7.2.3 s erine as partate orotate 1.3.1.14 2.4.2.10 2.7.4.6 triphos phate triphos phate
1.1.1.29 (UT P ) (C T P )
C OO
HO HO
C OO ATP 2.6.1.52
2.6.1.22
NH 2 NH 2
C OO H H H H H H H H H H C ONH 2
+ C N OC N N H H
γ-L inolenate
1.14.99.25 1.13.11.34 P OC H 2 C C C C N C CH P OC H 2 C C C C NH C CH P OC H 2 C C CO C H2 NH C CH
P OC H2C HOH C OO
L inoleate A rac hidonate L eukotriene B 4 OH 1.1.1.95
P OC H 2 C OC OO
HC C HC C HC C P OC H 2 C C C CH
C OO 5. O
3-P -G lyc erate P -Hydroxy- OH OH
N N OH OH
N N OH OH
N N
1 .1 3 . 9 9 C OO O R P (P P ) O RP RP OH OH HN N
1.3.1.35 4 .9 . 3
C OO pyruvate 3.6.1.31 3.5.4.19 C
9 .1 O 2.4.2.17
P -R ibos yl-A T P P -R ibos yl-A MP P -R ibos ylformimino P -R ibulos ylformimino Imidazole H
C OS C oA
L C O-S -AC P
HO OH 5.3.99.5
HO
OH 2.7.1.31 5-aminoimidazole- 5.3.1.16 5-aminoimidazole- glyc erol-P
HC C C H 2 C HC OO
I Oleoyl-C oA P almitoleoyl-A C P P ros taglandin P G E2 T hromboxane B 2 P OC H2 C H(O P ) C OO + c arboxamide-R P +
c arboxamide-R P+
HOC H2C H(OH) C OO N NH NHC OC H 2 C H 2 NH 2 HC C C H 2 C H(NH 3 )C OO + HC C C H 2 C OC H 2 OP
1.14.99.5 2, 3-Diphos pho- 5.4.2.1 HC C C H 2 C H(NH 3 )C HO HC C C H 2 C H(NH 3 )C H 2 OH HC C C H 2 C H(NH 3 )C H 2 OP
P C OS C oA C H 3 (C H 2 ) 14 C H=C HC OS -C oA C H 3 (C H 2 ) 14 C H(OH)C H 2 C OS -C oA C H 3 (C H 2 ) 14 C OC H 2 C OS -C oA
glyc erate G lyc erate C
H C arnos ine N
CH
NH
N NH N NH N NH
N
C
NH
4.2.1.19
I S tearoyl-C oA Dehydros tearoyl-C oA OH-S tearoyl-C oA Oxos tearoyl-C oA C H 3C OO
CH CH CH H
C H 3 (C H 2 ) 14 C OC H 2 C OS -C oA
HIS T IDINE His tidinal His tidinol His tidinol-P Imidazole
D E ndoplas mic R etic ulum ACE TATE 4.1.1.22 1.1.1.23 1.1.1.23 3.1.3.15 2.6.1.9
ac etol-P
C H 3 (C H 2 ) 14 C OS -AC P C H 3 (C H 2 ) 14 C OS C oA HOC H2C H(O P ) C OO HC C C H 2 C H 2 NH 2
4.3.1.3
C hain elongation Mitoc hondrial
P almitoyl-A C P P almitoyl-C oA 2-P -G lyc erate OOC C HC H 2 C H 2 C OO OC C HC H 2 C H 2 C OO CH C CH C HC OO
B C H 3 (C H 2 ) n C H=C HC OS -C oA C H 3 (C H 2 ) n C H(OH)C H 2 C OS AC P C H 3 (C H 2 ) n C OC H 2 C OS AC P
1.2.1.4
N
C
NH
HN NH N NH N NH 4.3.1.3
I 1.3.1.9 4.2.1.60 1.1.1.100
H HIS T A MINE CH
3.5.2.7 CH
4.2.1.49 CH
A C Y L -A C P 2, 3-E noyl-A C P 4.2.1.61
3-OH-A c yl-A C P 3-Oxoac yl-A C P C H 3 C H 2 OH
F ormimino Imidazolone Uroc anate
O 1.3.1.10 2.3.1.41
4.2.1.11 E T HA NOL 2 .3
glutamate propionate
2.1.3.2
S C H 3 (C H 2 ) 5 C H=C HC H 2 C OS AC P
4. 2. 1.
.1 .3
0
C H 3 (C H 2 ) 6 C H 2 C H 2 C OS AC P 3, 4-Dec enoyl-A C P 60 C H 3 (C H 2 ) 6 C H(OH)C H 2 C OS AC P C H 3 (C H 2 ) 6 C OC H 2 C OS AC P +
Y Dec anoyl-A C P 1. 3. 1. 3-OH-Dec anoyl-A C P
1.1.1.100
3-Oxo-Dec anoyl-A C P 1.1.1.1
C H 2 C O-OC H 2 C H(NH 3 )C OO
HS HS O - P hos phoadenylyl- A denylyls ulphate 2-
SO 4
9 C H 3 (C H 2 ) 6 C H=C HC OS AC P A c etyls erine
N 2, 3-Dec enoyl-A C P 4 .2 .1
.6 0 2.3.1.41 C H 2 =C (O P ) C OO C H 3 C HO +
1.8.99.1 1.8.99.2
s ulphate 2.7.1.25 (A P S ) 2.7.7.4
(P A P S )
T C H 3 (C H 2 ) 2 C H 2 C H 2 C OS AC P C H 3 (C H 2 ) 2 C H=C HC O-S -AC P C H 3 (C H 2 ) 2 C H(OH)C H 2 C OS AC P C H 3 (C H 2 ) 2 C OC H 2 C OS AC P P -enolpyruvate A c etaldehyde
S -C H 2 C H(NH 3 )C OO
+
4 .2
.9 9 +
+
C H 2 C H(NH 3 )C OO +
1.1.1.100 .8 HS C H 2 C H(NH C H 3 S C H C H C H(NH )C OO
H Hexanoyl-A C P
1.3.1.9
2, 3-Hexenoyl-A C P
4.2.1.59
3-OH-Hexanoyl-A C P 3-Oxo-Hexanoyl-A C P
HS
S -C H 2 C H(NH 3 )C OO 3 )C OO
4.4.1.1
+
S C H 2 C H 2 C H(NH 3 )C OO 4.2.1.22
+
HS C H 2 C H 2 C H(NH 3 )C OO
2.1.1.13
2 2 3
1.2.1.32
2.3.1.41
E C H 3 C H=C HC O.S -AC P C Y S T INE 1.6.4.1 C Y S T E INE
4.2.99.9
C ys tathionine 4.4.1.8
Homoc ys teine 2.1.1.14
ME T HIONINE
S C H 3 C H 2 C H 2 C OS AC P
1.3.1.9
C H 3 C H=C HC O-S -AC P
4.2.1.58
C H 3 C H(OH)C H 2 C OS -AC P
1.1.1.100
C H 3 C OC H 2 C OS AC P A DP .1 .1 1.13.11.20 Adenos yl
4 .1 4.4 .1.1 5 3.3.1.1 2.5.1.6
I B utanoyl-A C P C rotonoyl-A C P 3-OH-B utanoyl-A C P
C H 2 OH C H 2 OH
A c etoac etyl-A C P HOOC C H 2 C O-S -AC P
Malonyl-A C P 2.7.1.40 G lutamate
Adenos yl
+ +
C H 3 S C H C H C H(NH )C OO
+ S C H 2 C H 2 C H(NH 3 )C OO 2 2 3
S R -C H 2 C OO
HOC H HOC H
1.1.1.8 2.3.1.41
HO 2 S C H 2 C H(NH 3 )C OO
+
HO 3 S C H 2 C H(NH 3 )C OO
S -A denos yl
+
S -A denos yl
C H 3 (C H 2 ) n+2 C OS -C oA ATP 4.4 .1. 15 HS O 3- C ys teine C ys teate 2.1.1.10
6.2.1.3 3.1.2.20 F A T T Y A C ID C H 2 OH C H 2O P
2.3.1.39 .1 .2 s ulphinate 4 .1 6 .3 .2 homoc ys teine 2.1.1.20 methionine
A C Y L -C oA G lyc erol
2.7.1.30
3-P -G lyc erol K E TONE B ODIE S
3 .7 HO 2 S C H 2 C OC OO .1 .2
9
.2 +
C H 2S H (S A M)
(C ytos ol) 2.3 .1.1 5 3-S ulphinyl OOC C H(NH 3 )C H 2 C H 2 C ONHC HC OO
2.3. 1.51 C H 3 C OC H 3 C H 3 C H(OH)C H 2 C OO
HOOC C H 2 C O-S C oA P Y R UV A T E 1.4.1 .1 + pyruvate HO 2 S C H 2 C H 2 NH 2
1.8.1.3
HO 3 S C H 2 C H 2 NH 2 γ-G lutamylc ys teine
2.3.1.7
C H 2 O-C O-R A c etone 3-OH-B utyrate Malonyl-C o-A 1.2.4.1
2.6.1.2 C H 3 C H(NH 3 )C OO Hypotaurine T aurine G lyc ine
C arnitine 3.1.1.3 C H 2 O-C O-R C H 2 O-C O-R 2.3.1.12 A L A NINE 4.1.1.29 C H 2S H
2.7.8.5 4.1.2.5 + 6 .3 .2 +
R ’-C O-OC H .3
L O-A c yl-c arnitine C H 2 O-C O-R "
R ’-C O-OC H R ’-C O-OC H 4.1.1.4 1.1.1.30
C H 3 C O-S -AC P 4.1.1.9
1.8.1.4 4. 1. 3.
18
OOC C H 2C H 2 C OO C H 2 C H 2 C H(NH 3 )C OO
S uc c inylhomos erine
OOC C H(NH 3 )C H 2 C H 2 C ONHC HC ONHC H 2 C OO
G lutathione
C H 2 OH 4.1.1.12
I T riac ylglyc erol Diac yl
C H 2O P C H 3 C OC H 2 C OO
A c etoac etate
A c etyl-A C P 1 .1 +
C H 3 C H(OH)C H(NH 3 )C OO + + B ile A c ids
P O-A c yl-c arnitine FAT 2.3.1.20 glyc erol 3.1.3.4 2.7.1.107
P hos phatidate .1 .2
7
2.6.1.18 P OC H 2 C H 2 C H(NH 3 )C OO HOC H 2 C H 2 C H(NH 3 )C OO
3.1.2.11 2.3.1.38 O-P hos pho- Homos erine
T HR E ONINE HOC H 2 C (C H 3 ) 2 C OC OO
I 3.1.1.28 CH3COCOO- C H 3 C H(OH)C OO
4.2.99.2
homos erine
2.7.1.39 2.3.1.46 HC HO
4.1.2.12
Oxopantoate
D C H 3 (C H 2 ) n C H 2 C H 2 C OS C oA P Y R UV A T E LACTATE OHC C H 2 C OO -
4. 1. 3.
18 C H3 C H3
C H3
+
A C Y L -C oA C H 3 (C H 2 ) n C H=C HC OS C oA
4.2.1.17
C H 3 (C H n C H(OH)C H 2 C OS C oA
1.1.1.35
C H 3 (C H 2 ) n C OC H 2 C OS C oA 4.
2. Malonic
C OO
1.1.1.3
C (OH)C H(OH)C OO C HC OC OO
C HC H(NH 3 )C OO 1.1.1.169
(Mitoc hondria) 1.3.99.3
2, 3-E noyl-C oA 3-OH-A c yl-C oA 3-Oxoac yl-C oA 2. 3. 1. NA D HOH 4.2.1.16 C H3
D 16
1.2.4.1
1.
52 s emi- C H 3 C OC (OH)C H 3
2-A c etolac tate
1.1.1.86
C H3
4.2.1.9
C H3 2.6.1.32
HOC H 2 C (C H 3 ) 2 C H(OH)C OO
C H 3 (C H 2 ) 2 C OC H 2 C OS C oA ATP GDP 2-3-Dihydroxy 2-Oxo- 1.4.1.8 V A L INE
E C H 3 (C H 2 ) 2 C H 2 C H 2 C OS C oA
Hexanoyl-C oA 1.3.99.3
C H 3 (C H 2 ) 2 C H=C HC OS C oA
2, 3-Hexenoyl-C oA
4.2.1.17
C H 3 (C H 2 ) 2 C H(OH)C H 2 C OS C oA
3-OH-Hexanoyl-C oA
1.1.1.35
3-Oxohexanoyl-C oA 2. 3. 1.
16 C O2
2.3.1.12
3.1.3.43 1.2.1.18 aldehyde C H 3 C H 2 C OC OO is ovalerate is ovalerate
P antoate
C O2 Oxobutyrate ß-A lanine
G 6.4.1.1 4.1.1.32
C H3 C H3 C H3
1.2.1.25
3.5.1.22
6.3.2.1
C H 3 C H 2 C H 2 C OS C oA C H 3 C H=C HC OS C oA C H 3 C H(OH)C H 2 C OS C oA C H 3 C OC H 2 C OS C oA C H3
R B utanoyl-C oA
1.3.99.2
C rotonoyl-C oA
4.2.1.55
3-OH-B utanoyl-C oA
1.1.1.157
A c etoac etyl-C oA
2.3 .1.9
NADH+H+ HOC H 2 C HC OO HOC H 2 C HC OS -C oA- C H 2 = C C OS C oA C H 3 C HC O-S C oA HOC H 2 C (C H 3 ) 2 C H(OH)C O NHC H 2 C H 2 C OO
3.1.2.4
A Odd C F atty ac ids CH3COSCoA
GTP
C H3
4 .1
.3 .1
8
3-Hydroxy- 3-Hydroxy- 4.2.1.17 Methyl 1.3.99.3 Is obutyryl-C oA P A NTOT HE NA T E
D C H 3 C H 2 C H 2 C H 2 C OS C oA C H 3 C H 2 C H=C HC OS C oA C H 3 C H 2 C H(OH)C H 2 C OS C oA C H 3 C H 2 C OC H 2 C OS C oA
4.1.3.5 A C E T Y L -C oA 31 is obutyrate Is obutyryl-C oA ac rylyl-C oA
P entanoyl-C oA P entenoyl-C oA 3-OH-P entanoyl-C oA
1.1.1.35
3-Oxopentanoyl-C oA NAD+ OHC C HC OO 1. 1. 1. 2.7.1.33
A 2.6 .1.4 4
Methylmalonyl
C H3 C H3
CH3
+
T 2.6 .1.4 s emialdehyde C OO C HC OC OO
C H C H(NH 3 )C OO P OC H 2 C (C H 3 ) 2 C H(OH)C O NHC H 2 C H 2 C OO
C (OH)C H(OH)C OO
C H 3C H 2 4-P -P antothenate
I C H 2 O-C O-R C H 2 O-C O-R C H 2 O-C O-R OHC C OO 4.1.3.5 4.1.3.4
1.1.1.39 C H 3 C OC (OH)C H 2 C H 3
4.2.1.9
C H 3C H 2 C H 3C H 2 2.6.1.32 C ys teine
OH OH R '-C O-OC H 2.7.7.41 G lyoxylate 2-A c eto-2- 2: 3-Di-OH- 2-Oxo-3-methyl IS OL E UC INE
6.3.2.5
O R '-C O-OC H O O C OO
+
R '-C O-OC H O
C H 2 C OO
-OOCCOCH COO-
2 2. 3. 1.
16
hydroxy- 1.1.1.86 3-methylvalerate valerate
C OO
C H 2 O P O C H 2 C HNH 3 1.1.1.79 NA DH+H + OX A L OA C E T A T E 2.6.1.1
P OC H 2 C (C H 3 ) 2 C H(OH)C O NHC H 2 C H 2 C O NHC HC H 2 S H
N C H 2 O-P O 1.3.99.7
- S erine
C H 2 O P OC MP
1.2.3.5
C H 3 C (OH)C H 2 C OS C oA
butyrate 4-P -P antothenylc ys teine
O - HO OH O
2.7.8.8
O
ß-OH-ß-Methyl- 4. 2. 1.
4.1.3.7
CH2COO- C H3 CH3 CH3 1.2.1.25
P HOS P HA T IDY L C DP -diac yl HOC H 2 C OO 18 NAD+ C H3
OH
Inos itol G lyc olate glutaryl-C oA 4.1.3.8 C(OH)COO- C H 3 C OC HC OS C oA C H 3 C H(OH)C HC OS C oA C H 3 C H=C HC OS C oA C H 3 C H 2 C HC OS C oA 4.1.1.36
S E R INE 2.7.8.11 glyc erol CH2COO-
P hos phatidyl 2-Methylac eto-1.1.1.35 2-Methyl-3-4.2.1.17T iglyl-C oA 2 Methylbutyryl-
inos itol C H 2 O-C O-R
1.2.1.21 HOOC -C OOH
1.1.1.34
C IT R A T E ac etyl-C oA hydroxy- 1.3.99.3
C oA P OC H 2 C (C H 3 ) 2 C H(OH)C O NHC H 2 C H 2 C O NHC H 2 C H 2 S H
C H 2 O-C O-R C H 2 O-C O-R ’ Oxalate F MN I 4.1.3.2
R '-C O-OC H O O HC O-C O-R R '-C O-OC H O 2.7.8.5 HOC H 2 C HO
I -OOCCHO 4.2.1.3
C H 3 C H 2 C OS C oA
butyryl-C oA 4-P -P antetheine
P C H 2 O-C O-R
4.1.1.65 C H 2 O-P O C H 2 C H(OH)C H 2 O-P -OC H 2 C H 2 O-P O C H 2 C HOHC H 2 OH G lyc ol Glyoxylate CH(OH)COO -
P ropanoyl-C oA 2.7.7.3
- - O- aldehyde C H 2 C OO Cycle CHCOO-
1.6.5.3
O
H R '-C O-OC H O
+ C ardiolipin
O
P hos phatidylglyc erol C H 3 C (OH)C H 2 C HO
1.1.1.37
CH2COO- 2.1.3.1 6.4.1.3 C OOH C OOH +
(C H 3 ) 2 C HC H 2 C H(NH 3 )C OO
F MNH 2 4.1.1.41
O C H 2 O P OC H 2 C H 2 NH 3
-
+ +
1.4.3.8 Mevaldate IS OC IT R A T E 5.1.99.1
(C H 3 ) 2 C HC (OH)C H 2 C OO (C H 3 ) 2 C HC HC H(OH)C OO (C H 3 ) 2 C HC H 2 C OC OO 2.6.1.6
L E UC INE
ADP - OC H 2 C (C H 3 ) 2 C H(OH)C O NHC H 2 C H 2 C O NHC H 2 C H 2 S H
Dephos pho-C oenzyme A
O C P P - OC H 2 C H 2 NH 3 P OC H 2 C H 2 NH 3 + 2-Is opropyl- 3-Is opropyl- 1.1.1.85 Oxoleuc ine
S 2.7.8.1 C DP -E thanolamine 2.7.7.14
E thanolamine-P 2.7 .1. 82
+
HOC H 2 C H 2 NH 3 2H 4.1.3.1 1.1.1.41 C H3 malate 4.2.1.33
malate 1.4.1.9
P hos phatidyl E thanolamine 1.1.1.32
2F e -S 2.7.1.24
P ethanolamine
C H 2 OH
CH3CH(OH)CH2CO.SCoA
OOC -C H-C OS C oA C H3 1.2.1.25
C H 2 O-C O-R (5 C lusters) MA L A T E 4.2 .1. 18 C H3
H C E P HA L IN HOC H O
HOC H O
+ C H 3 C OC H 2 C H 2 N(C H 3 ) 3 -OOCCOCH CH COO-
4.1.1.71
Methylmalonyl-C oA OOC C H 2 C = C HC OS C oA C H 3 C = C HC OS C oA (C H 3 ) 2 C HC H 2 C OS C oA P -ADP - OC H 2 C (C H 3 ) 2 C H(OH)C O NHC H 2 C H 2 C O NHC H 2 C H 2 S H
A
C H 2 C OO
4H+ 6.4.1.4 1.3.99.10
2 2
O + C H 2 OP O C H 2 C H 2 N(C H 3 ) A c etylc holine 4H+ 2-OXO - 3-Methyl- 3-Methyl- Is ovaleryl-C oA C oenzyme A
2.1.1.17
C H 2 OP O C H 2 C H 2 N(C H 3 ) 3
3.1. 1.5
-
G lyc erophos phoc holine
O
3 .1
C H 3 C (OH)C H 2 C H 2 OH 1.6. 5.3 AS PAR TATE 2 .7 .2 4.2.1.18
glutac onyl-C oA c rotonyl-C oA M
L C H 2 OC H=C HR 2.1.1.71 O - L ys olec ithin .4 .2 Mevalonate 2H+ 4.2.1.2 G L UT A R A T E .4
1.1.1.3
C H 2 O-C O-R 3.1.4.4 2.3.1.6 or 1.2.4.2 I
I R -C O-OC H O
+ R '-C O-OC H O
+
3.1.1.32
3.1.4.3 2.7.1.36
2H+ -
2.3.1.61 +
P OOC C H 2 C H (NH 3 )C OO N
C H 2 OP O C H 2 C H 2 N(C H 3 ) 3 2.7.4.2 OOCCH=CHCOO-
P - O
C H 2 OP O C H 2 C H 2 N(C H 3 ) 3
O -
+
C P P -O C H 2 C H 2 N(C H 3 ) 3
+
OC H 2 C H 2 N(C H 3 ) 3
+
HOC H 2 C H 2 N(C H 3 ) 3 F UMA R A T E
4.3.1.1
A s partyl-P 1 .2
H
C
H2
C
OOC C H 2 C H 2 C ONH
+
NH 3
O
I C holine L E C IT HIN C DP -c holine 2.7.7.15
C holine-P C HOL INE C H 2 C OO UQH 2 6.3.5.4 .1 .1
1 +
HC C H2
C H-C OO
H 2C C H2
C H-C OO
OOC C H 2 C H 2 C ONH OOC C HC H 2 C H 2 C H 2 C H-C OO
+
OOC C H-C H 2 C H 2 C H 2 C HC OO
+
plas malogen 1.3.1.35 2.7.8.2 2.7.1.32 OOC C OOC C
D 2 .7 .8
.3
3.1 .4. 12 C H 3 C (OH)C H 2 C H 2 O P P F ADH 2 +
-OOCCH2CH2CO.SCoA 5.1.99.1 OHC C H 2 C H (NH 3 )C OO N
1.3.1.26
N OOC C OC H 2 C H 2 C H 2 C H-C OO NH 3 NH 3
S erine +NH + + + Diphos pho- S UC C INY L -C oA 5.4.99.2 OOC C H 2 C H 2 C HO A s partyl 4.2.1.52 2, 3-Dihydro- P iperideine- N-S uc c inyl- 2.6.1.17
N-S uc c inyl-2, 63.5.1.18 Diamino- A
S 2.3.1.50 3 NH 3 NH 3 NH 3
mevalonate
F e-S 1.3.5.1
6.2.1.4 S uc c inic S emialdehyde dipic olinate 2, 6-dic arboxylate 2-amino-6-oxo- diaminopimelate pimelate C
C H 3 (C H 2 ) 14 C OC HC H 2 OH C H 3 (C H 2 ) 14 C H(OH)C HC H 2 OH C H 3 (C H 2 ) 12 C H=C HC H(OH)C HC H 2 OH C H 3 (C H 2 ) 12 C H=C HC H(OH)C HC H 2 O- G alac tos e 1.2.1.16
s emialdehyde pimelate
Dehydros phinganin
1.1.1.102
S phinganin 4-S phingenin P s yc hos ine C yt.b F AD
-OOCCH2CH2COO-
S UC C INA T E
OH
+ + + + + + 5 .1 .1
.7 I
H 2 N(C H 2 ) 4 C H(NH 3 )C OO
G anglios ides UDP -S ugars A c yl-C oA
2.4.1.23 UQ II Glycine (C H 3 ) 3 NC H 2 C H(OH)C H 2 C OO (C H 3 ) 3 N(C H 2 ) 3 C H 2 C H(NH 3 )C OO (C H 3 ) 3 N(C H 2 ) 3 C H 2 C H(NH 3 )C OO
4. 1. 1.
20
D
2.4 .1. 62 3.5.1.23 A c yl-C oA 4.1.1.33 2.3.1.37 + C arnitine N 6 -T rimethyl- N 6 -T rimethyllys ine LY S INE 1.5.1.7 - 10
UDP -G alac tos e UQH 2 2H+
H 2 NOC C H 2 C H (NH 3 C OO
1.14.11.1
3-OH-lys ine
1.14.11.8 S
NHAcyl O
+ NHC OR
NHC OR A s paragine 2.6.1.19 C OO
C H 3 (C H 2 ) 12 C H=C HC H(OH)C HC H 2 O P O C H 2 C H 2 N(C H 3 ) 3
O - C H 3 (C H 2 ) 12 C H=C HC H(OH)C HC H 2 OH C H 3 (C H 2 ) 12 C H=C HC H(OH)C HC H 2 O- G alac tos e C H2 5-A mino- +
NH C HC H 2 C H 2 C OO
+ +
S P HING OMY E L IN
2.7.8.3
C eramide C erebros ide C H 3 C -C H 2 C H 2 O P P levulinate 1.3.99.7 OOC C H 2 C H 2 C H 2 C OS C oA OOC C H 2 C H 2 C H 2 C OC OO OOC C H 2 C H 2 C H 2 C H (NH 3 ) C OO OHC C H 2 C H 2 C H 2 C H (NH 3 ) C OO C H 2 C H 2 C H 2 C H 2 C H (NH 3 ) C OO
3.1.4.12 3.2.1.46 2.4.1.47 2UQH 2 UQH 2
1.3.99.7 Is opentenyl-P P 1.10.2.2 + 4.1.1.70
G lutaryl-C oA 2-Oxoadipate 2.6.1.39
2-A minoadipate 1.2.1.31 2-A minoadipate 1.5.1.9
S ac c haropine
R -C H(NH 3 ) C OO
C H3
(C 5) 4H+ OOC C H 2 C H 2 C H 2 NH 2 s emialdehyde
I 2H+ 2-A MINO A C ID A denos yl
L yc opene P hytoene
C H 3 C = C HC H 2 O P P
2e- 2UQ _. 4-A minobutyrate
S (C 40) Dimethylallyl-P P III 1e- _ R -C O-C OO (G A B A ) H 2 N(C H 2 ) 3 NH (C H 2 ) 4 NH (C H 2 ) 3 NH 2
C H 3 -S C H 2 C H 2 C HNH 2
+
(C 40) (C 5) 2-OXO A C ID S permine S -A denos ylmethyl
UQ.
TR A
T o B rain -V IS ION 2.5.1.1 2e- 15
O hv CH3 C H3 F e-S +
OOC C H 2 C H 2 C H (NH 3 ) C OO 1.
1. thiopropylamine L E G E ND
2.6.1.- 4.
NS
P ß-C A R OT E NE (C 40) 2.5.1.32 2.5.1.29 C H 3 C = C HC H 2 C H 2 C = C HC H 2 O P P C yt.bL C yt.bH
1e-
MI
(Dec arboxylated S A M)
A
R Metarhodops in R hodops in O
C H 2O P P G eranyl-P P NA
T IO N G L UT A MA T E 2. 7. 2. +
2.5.1.22 C arbohydrates A mino A c ids
11 P OOC C H C H C H (NH ) C OO
E 1.13.11.21 C H 3O C H3
G eranyl-geranyl-P P (C 10) C yt.c 1 2 2 3 H 2 N(C H 2 ) 4 NH (C H 2 ) 3 NH 2
B ios ynthes is B ios ynthes is
C OO - Ops in C H 3O (C 20) G lutamyl-P S permidine Degradation Degradation
N n
2UQ UQ
R etinoate
O Ubiquinone 2.5.1.10 1.10.2.2
O 1.2.1.36
C HO (C oenzyme Q) OPP C yt.c 1.4.1.2 1.4.1.14 3.5.1.2
6.3.1.2 1.2.1.41
L ipids P urines &
C H2 2.5.1.16
I trans -R etinal 5.2.1.3
11-c is -R etinal C HO Menaquinone C H 2 OH B ios ynthes is P yrimidines
D
L ight P hytol (C 20) ting A
s por . 6 . 1 . 3 T P s y NO 3 - NH 4+ +
H 2 NOC C H 2 C H 2 C H (NH 3 ) C OO + H 2 NC H 2 C H 2 C H 2 C H 2 NH 2
Degradation B ios ynthes is
1.1.1.105 1.1.1.105 n
R etinol es ters P las toquinone C uA C uA +-
tra 3 4 nth 1.6.6.1 G lutamine OHC C H 2 C H 2 C H (NH 3 ) C OO
P utres c ine Degradation
S C H3
F arnes yl-P P os cp 1.7.99.4 .7.1
1.7 .6.4 ATP G lutamic
as
O
H
HO CH3
e
1.6 .1 s emialdehyde
NO 2 -
2.3.1.76 (C 15) C O2
3.1.1.21
C H 2 OH
C H 2 OH
CH3 O
C H3 Heme a 2H+ 1.
18
.6
6.3.4.16 C H 3 C OC OO OHC C OO
V itamins C o-enzymes & Hormones
B ios ynthes is Degradation
S 5.2.1.7 6.3.5.5 C H2 C H2
5
trans -R etinol 11-c is -R etinol O
P hylloquinone α-T oc opherol
5.
δ F1 P yruvate G lyoxylate
3.
2.5.1.21
T Dark N2
6.
(V itamin A ) (V itamin K ) (V itamin E ) F6
CH C HC OO
H 2 NC OO P 2.6.1.13 P entos e P hos phate P athway
E S T E R OIDS 2H+ C uB Heme a 3 C arbamoyl-P +
H 2 NC ONHC H 2 C H 2 C H 2 C H (NH 3 ) C OO
N
P yrroline-5-
2H + AT P
R 2
β ATP C IT R UL L INE c arboxylate
4.1.3.16
P hotos ynthes is Dark R eac tions
2e- γ
O P roges terone
3
β
OOC C H(OH)C H 2 C OC OO
AD
β 2.1.3.3 4.1.1.17
4-Hydroxy-
P
H
AαT 1.5.99.8
H H 1.9.3.1
I HO HO HO HO
Pi
P +1 P i α 1.5.1.2
C H2 C H2
2-oxoglutarate
Human Metabolis m is identified as far pos s ible by black arrows
IV AD P +
H B ios ynthes is Degradation
D P regnenolone C HOL E S T E R OL Des mos terol Zymos terol
H
L anos terol 5.4.99.7
S qualene
1/ O
2 2 H+
H+
+
H 2 NC H 2 C H 2 C H 2 C H (NH 3 ) C OO
2.1.3.3
C H2 C HC OO
1.14.99.7
S (C 30) H+
H+
β
F1 OR NIT HINE
NH
2.6.1.23 C OMP A R T ME NT A T ION
γ 6.3.4.5
P R OL INE
HE MOG L OB IN C HL OR OP HY L L H+
C OO -
C OO -
H2O 1.14.11.2
+
OOC C H(OH)C H 2 C H(NH 3 )C OO
T he "B ackbone" of metabolis m involves
ε H 2 NC ONH 2 OOC C HC H 2 C OO G LY C OLY S IS in the C Y T OP LAS M,
P C H2
C H3
C H2 C H2
C OO - C H2 C OO -
C OO -
COO-
CH2
δ F0 2.1.4.1 UR E A N + HOC H C H2
4-Hydroxy- the T C A C Y C LE (mainly) in the Mitochondrial matrix
O CH H CH H2 C H3 C H C H2 H2 C H3 H+ H 2 NC NHC H 2 C H 2 C H 2 C H (NH 3 ) C OO glutamate
TR A NS L O
CH 2 C H2 - OOC C H2 H2 C H2 CH2
and AT P F OR MAT ION s panning the
C C C C H2 a H+ H+ G lyc ine + 3.5.3.1 A rginino- H 2C C HC OO
MIT OC HONDR IAL INNE R ME MB R ANE
R H 3C CH H 3C CH H3C C H2
H 2C
C
C H2
H2NCH2C=O
α
H+ H+
s NH 2
3.5.3.6
NO s uc c inate
N
H 1.5.1.12
HOC H C H2
N N N N N N 5-A mino- n it An electron flow (an electric current) generated from
P HC Fe CH
H H H H N
H H
N
C OO levulinate
-
1 0 c -s ucb- u +
H 2 NC NHC H 2 C H 2 C H 2 C H (NH 3 ) C OO 1.14.13.39 HC C HC OO
NADH and UQH2 drives the translocation of protons
H
H2C
H H
C H2 H 2C
H H
C H2
H 2C C H2
A HY DR OXY 3-Hydroxy-
N
from the matrix to the intermembrane space.
C
N N N N N N
N
H H
N
- OOC
CH 2 TE
D P R OTONS H+ H+ A R G ININE P R OL INE pyrroline-
The retrolocation of these protons through the F0 subunits
Y H3C
C
C H3 H 3C
C
C H3 H 3C
C
C H3
H 2C C H2 CH 2 4.2.1.24 H+ H+ of ATP synthase to the matrix then supplies the energy
H 2C 4.3.2.1 5-c arboxylate
R C H2
H C H2 C H2 H2 C H2 C H2 H2 C H2
-
OOC C H2
C
H2 C H2
C OO - A DP Pi
+ NH + NH 2 + NH 2 HN C
NH CO
needed to form ATP from ADP and phosphate
I C H2
C OO -
C H2
C OO -
C H2
C OO -
C H2
C OO -
C H2
C OO -
C H2
C OO -
C H2 C H2 H 2C N
H X ATP Y
2
H 2 NC NHC H 2 C OO H 2 NC N(C H 3 )C H 2 C OO P - HNC N(C H 3 )C H 2 C OO
N CH2
1.5.1.2
E lectron F low P roton F low
C OO - C OO -
N H2N
G uanidoac etate
2.1.1.2
C reatine 2.7.3.2 P -C reatine
C H3
S mall Numbers ( eg. 2.4.6.7) refer to the IUB MB E nzyme
1.3.3.4 1.3.3.3 4.1.1.37 4.3.1.8 C reatinine
S HE ME P rotoporphyrinogen C oproporphyrinogen Uroporphyrinogen P orphobilinogen
E ND
E R G O N IC R E A C T IO N
3.5.2.10 C ommis s ion (E C ) R eference Numbers of E nzymes
4.99.1.1 4.2.1.75
© 2003 International Union of Biochemistry and M olecular Biology 22 nd Edition Designed by Donald E. Nicholson, D.Sc., The University of Leeds, England – and Sigma-A ldrich
Product No. M 3907
www.iubmb.org
A rgentina Brazil Finland India Italy The Netherlands Russia Sw eden United States
SIGMA-ALDRICH DE SIGMA-ALDRICH BRASIL LTDA. SIGMA-ALDRICH FINLAND SIGMA-ALDRICH CHEMICALS SIGMA-ALDRICH S.r.l. SIGMA-ALDRICH CHEMIE BV SIGMA-ALDRICH RUSSIA SIGMA-ALDRICH SWEDEN AB SIGMA-ALDRICH
ARGENTINA, S.A. Tel: 55 11 3732-3100 Tel: 358-9-350-92 50 PRIVATE LIMITED Telefono: 02 33417310 Tel Gratis: 0800-0229088 TechCare Systems, Inc. Tel: 020-350510 P.O. Box 14508
Tel: 54 11 4556 1472 Fax: 55 11 3733-5151 Fax: 358-9-350-92 555 Telephone Fax: 02 38010737 Fax Gratis: 0800-0229089 (SAF-LAB) Fax: 020-352522 St. Louis, Missouri 63178
Fax: 54 11 4552 1698 Bangalore: 91-80-5112-7272 Numero Verde: 800-827018 Tel: 078-6205411 Tel: 095-975-1917/3321 Outside Sweden Tel: +46 8 7424200 Toll-free: 800-325-3010
Canada France Hyderabad: Fax: 078-6205421 Fax: 095-975-4792 Outside Sweden Fax: +46 8 7424243 Call Collect: 314-771-5750
A ustralia SIGMA-ALDRICH CANADA LTD. SIGMA-ALDRICH CHIMIE S.à.r.l. 91-40-5531 5548 / 2784 2378 Japan Toll-Free Fax: 800-325-5052
SIGMA-ALDRICH PTY., LIMITED Free Tel: 800-565-1400 Tel appel gratuit: 0800 211 408 Mumbai: SIGMA-ALDRICH JAPAN K.K. New Zealand Singapore Sw itzerland Tel: 314-771-5765
Free Tel: 1800 800 097 Free Fax: 800-265-3858 Fax appel gratuit: 0800 031 052 91-22-2579 7588 / 2570 2364 Tokyo Tel: 03 5821 3111 SIGMA-ALDRICH PTY., LIMITED SIGMA-ALDRICH PTE. LTD. FLUKA CHEMIE GmbH Fax: 314-771-5757
Free Fax: 1800 800 096 Tel: 905-829-9500 New Delhi: Tokyo Fax: 03 5821 3170 Free Tel: 0800 936 666 Tel: 65-6271 1089 Swiss Free Call: 0800 80 00 80 Internet:
Tel: 612 9841 0555 Fax: 905-829-9292 Germany Free Fax: 0800 937 777 Fax: 65-6271 1571 Tel: +41 81 755 2828
SIGMA-ALDRICH CHEMIE GmbH 91-11-2616 5477 / 2619 5360 K orea sigma-aldrich.com
Fax: 612 9841 0500 Fax Fax: +41 81 755 2815
China Free Tel: 0800-51 55 000 SIGMA-ALDRICH KOREA Norw ay South A frica
A ustria SIGMA-ALDRICH CHINA INC. Free Fax: 0800-649 00 00 Bangalore: 91-80-5112-7473 Tel: 031-329-9000 SIGMA-ALDRICH NORWAY AS SIGMA-ALDRICH United K ingdom
SIGMA-ALDRICH HANDELS GmbH Tel: 86-21-6386 2766 Hyderabad: 91-40-5531 5466 Fax: 031-329-9090 Tel: 23 17 60 60 SOUTH AFRICA (PTY) LTD. SIGMA-ALDRICH COMPANY LTD.
Tel: 43 1 605 81 10 Fax: 86-21-6386 3966 Greece Mumbai: 91-22-2579 7589 Fax: 23 17 60 50 Tel: 27 11 979 1188 Free Tel: 0800 717181
Fax: 43 1 605 81 20 SIGMA-ALDRICH (O.M.) LTD New Delhi: 91-11-2616 5611 M alaysia Fax: 27 11 979 1119 Free Fax: 0800 378785
Czech Republic Tel: 30 210 9948010 SIGMA-ALDRICH (M) SDN. BHD Poland Tel: 01747 833000
Belgium SIGMA-ALDRICH s.r.o. Fax: 30 210 9943831 Ireland Tel: 603-56353321 SIGMA-ALDRICH Sp. z o.o. Spain
SIGMA-ALDRICH IRELAND LTD. Fax: 01747 833313
SIGMA-ALDRICH NV/SA. Tel: 246 003 200 Fax: 603-56354116 Tel: (+61) 829 01 00 SIGMA-ALDRICH QUÍMICA S.A.
Free Tel: 0800-14747 Fax: 246 003 291 Hungary Free Tel: 1800 200 888 Fax: (+61) 829 01 20 Free Tel: 900101376
Free Fax: 0800-14745 SIGMA-ALDRICH Kft Free Fax: 1800 600 222 M exico Free Fax: 900102028
Tel: 03 899 13 01 Denmark Tel: 06-1-235-9054 SIGMA-ALDRICH QUÍMICA, Portugal
SIGMA-ALDRICH Fax: 06-1-269-6470 S.A. de C.V. SIGMA-ALDRICH QUÍMICA, S.A.
Fax: 03 899 13 11
DENMARK A/S Ingyenes zöld telefon: 06-80-355-355 SIGMA-ALDRICH ISRAEL LTD. Free Tel: 01-800-007-5300 Free Tel: 800 20 21 80
sigma-aldrich.com/pathw ays
Tel: 43 56 59 10 Ingyenes zöld fax: 06-80-344-344 Tel: 08-948-4100 Free Fax: 01-800-712-9920 Free Fax: 800 20 21 78
XXX Fax: 43 56 59 05 Fax: 08-948-4200
You might also like
- The Soil Mites of the World: Vol. 1: Primitive Oribatids of the Palaearctic RegionFrom EverandThe Soil Mites of the World: Vol. 1: Primitive Oribatids of the Palaearctic RegionNo ratings yet
- Mark Kurt Schutze Thesis 2Document173 pagesMark Kurt Schutze Thesis 2Azhar Ahmad NasriNo ratings yet
- Field Identification Guide To The Sharks and Rays of The Mediterranean and Black SeaDocument11 pagesField Identification Guide To The Sharks and Rays of The Mediterranean and Black SeamorphelyaNo ratings yet
- Sharks and Rays of The Red Sea-2004Document91 pagesSharks and Rays of The Red Sea-2004Андрей БорщикNo ratings yet
- Bat Roosts in Rock: A Guide to Identification and Assessment for Climbers, Cavers & Ecology ProfessionalsFrom EverandBat Roosts in Rock: A Guide to Identification and Assessment for Climbers, Cavers & Ecology ProfessionalsNo ratings yet
- Insects Spiders and Mites of Cape Breton PDFDocument304 pagesInsects Spiders and Mites of Cape Breton PDFJumentoNo ratings yet
- Guide Identification of Seagrasses GBRDocument61 pagesGuide Identification of Seagrasses GBRRusydi Machrizal100% (1)
- Ikan Di Kepualauan Indo-AustraliaDocument480 pagesIkan Di Kepualauan Indo-AustraliaDediNo ratings yet
- Strandtmann & Wharton (1958) Manual of Mesostigmatid MitesDocument415 pagesStrandtmann & Wharton (1958) Manual of Mesostigmatid MitesMichelNo ratings yet
- Cronquist system diagramDocument22 pagesCronquist system diagramAnaVievietrotieenNo ratings yet
- The Soil Mites of the World: Vol. 3: Oribatid Mites of the Neotropical Region IIFrom EverandThe Soil Mites of the World: Vol. 3: Oribatid Mites of the Neotropical Region IINo ratings yet
- Marine Mammals of The World: Fao Species Identification GuideDocument5 pagesMarine Mammals of The World: Fao Species Identification GuideajlagunaNo ratings yet
- Key To Seed and Leaf Beetles of The British IslesDocument96 pagesKey To Seed and Leaf Beetles of The British IsleswilhelmNo ratings yet
- Fossil SpidersDocument36 pagesFossil SpidersCélio Moura NetoNo ratings yet
- Escarabajos Peloteros de Madre de DiosDocument3 pagesEscarabajos Peloteros de Madre de DiosWalter Guillermo Cosio LoaizaNo ratings yet
- Diptera Vol 1Document684 pagesDiptera Vol 1Aquiles Reyes100% (1)
- Plataspidae Related Cantharodes ReviewDocument33 pagesPlataspidae Related Cantharodes ReviewanilimNo ratings yet
- Komodo Facts BookDocument48 pagesKomodo Facts BookAndrew HarveyNo ratings yet
- Three New Cichlid Species From Southem Amazonia: And: Aequidens Gerciliae, A. Epae A. MichaeliDocument19 pagesThree New Cichlid Species From Southem Amazonia: And: Aequidens Gerciliae, A. Epae A. Michaeliceflemos1043No ratings yet
- FISHES of The WORLDDocument12 pagesFISHES of The WORLDkyywyy100% (1)
- 2009 The Rise of Amphibians - 365 Million Years of EvolutionDocument390 pages2009 The Rise of Amphibians - 365 Million Years of EvolutionLuiza PassosNo ratings yet
- Zooplankton Guide 27Document272 pagesZooplankton Guide 27Ratna AvitasariNo ratings yet
- EliotUpdatingButterfliesMalayPeninsula PDFDocument65 pagesEliotUpdatingButterfliesMalayPeninsula PDFOshLifin RucmanaNo ratings yet
- Rivero 1991. New Ecuadorean Colostethus (Amphibia, Dendrobatidae) in The Collection of The National Museum of Natural History, Smithsonian InstitutionDocument16 pagesRivero 1991. New Ecuadorean Colostethus (Amphibia, Dendrobatidae) in The Collection of The National Museum of Natural History, Smithsonian InstitutionNicole AcosVas0% (1)
- Guiadecampo CochaCashu Peces Manu PDFDocument4 pagesGuiadecampo CochaCashu Peces Manu PDFLuis Miguel Pineda CoronelNo ratings yet
- Gastropoda Bibliography (World-Freshwater), Christophe Avon 2015Document1,787 pagesGastropoda Bibliography (World-Freshwater), Christophe Avon 2015Christophe AvonNo ratings yet
- Plankton A Guide To Their Ecology and Monitoring For Water QualityDocument263 pagesPlankton A Guide To Their Ecology and Monitoring For Water QualityLumban Tobing Jonathan100% (1)
- 04-Coats and Clamp 2009-Ciliated Protists of The GoMxDocument24 pages04-Coats and Clamp 2009-Ciliated Protists of The GoMxebutaNo ratings yet
- Phylum Cnidaria WorksheetDocument4 pagesPhylum Cnidaria WorksheetMi RiveraNo ratings yet
- History of Navy Entomology 1941-2011Document35 pagesHistory of Navy Entomology 1941-2011rville1559No ratings yet
- AAFC Insects and Arachnids 17 Wolf Nurseryweb and Lynx Spiders of Canada and AlaskaDocument388 pagesAAFC Insects and Arachnids 17 Wolf Nurseryweb and Lynx Spiders of Canada and AlaskaCana DianNo ratings yet
- AVES de La Reserva PacayaDocument61 pagesAVES de La Reserva PacayaGianina J. Mundo PadillaNo ratings yet
- Snakes of EuropeDocument352 pagesSnakes of Europetobiasaxo5653100% (2)
- ArtemiaDocument17 pagesArtemiaRafael SalesNo ratings yet
- Beetles of the World_ A Natural History 2023 - Princeton University PressDocument242 pagesBeetles of the World_ A Natural History 2023 - Princeton University Pressfernando LEISNo ratings yet
- Thesis B Dealy - Piezogaster Generic RevisionDocument125 pagesThesis B Dealy - Piezogaster Generic RevisionBeau Dealy100% (2)
- Boats of The WorldDocument505 pagesBoats of The WorldGigi FrancoNo ratings yet
- Freshwater Turtles & Terrapins DemoDocument11 pagesFreshwater Turtles & Terrapins DemoSonny ToshiroNo ratings yet
- Australian Dragonflies: A Guide to the Identification, Distributions and Habitats of Australian OdonataFrom EverandAustralian Dragonflies: A Guide to the Identification, Distributions and Habitats of Australian OdonataRating: 3 out of 5 stars3/5 (1)
- Fishes of NoakhaliDocument325 pagesFishes of NoakhaliM. Shahadat Hossain50% (2)
- HIMI Benthic Invertebrate Field GuideDocument159 pagesHIMI Benthic Invertebrate Field GuideNita PuspitaNo ratings yet
- Guides To The Freshwater Invertebrates of Southern Africa Volume 10 - ColeopteraDocument277 pagesGuides To The Freshwater Invertebrates of Southern Africa Volume 10 - Coleopteradaggaboom100% (1)
- 2012 Libyan Desert Expedition 13 June 2012 FinalDocument35 pages2012 Libyan Desert Expedition 13 June 2012 FinalZulema Barahona MendietaNo ratings yet
- RADFORD Et Al Vascular Plant Systematics Chapter 6 Phytography PDFDocument70 pagesRADFORD Et Al Vascular Plant Systematics Chapter 6 Phytography PDFThomaz SinaniNo ratings yet
- Takhtajan - 1980 - Outline of The Classification of Flowering Plants (Magnoliophyta)Document135 pagesTakhtajan - 1980 - Outline of The Classification of Flowering Plants (Magnoliophyta)Paul Palacin Guerra0% (1)
- Atelopus Galactogaster Rivero & Serna, 1993 PDFDocument6 pagesAtelopus Galactogaster Rivero & Serna, 1993 PDFJuan Pablo AlfaroNo ratings yet
- Pro To Zoology 1954 KudoDocument988 pagesPro To Zoology 1954 KudoClaau GomezNo ratings yet
- Bulletins of Ameri 363 PaleDocument570 pagesBulletins of Ameri 363 PaleArturo Palma RamírezNo ratings yet
- Alaska FishesDocument31 pagesAlaska FishesHema LaughsalotNo ratings yet
- Aggsbach - de Abensberg Arnhofen Flint PlattensilexDocument2 pagesAggsbach - de Abensberg Arnhofen Flint PlattensilexAnonymous y51HfcYUgNo ratings yet
- Guides To The Freshwater Invertebrates of Southern Africa Volume 2 - Crustacea IDocument136 pagesGuides To The Freshwater Invertebrates of Southern Africa Volume 2 - Crustacea IdaggaboomNo ratings yet
- Drug 1Document2 pagesDrug 1Nicholas TagleNo ratings yet
- F4D 24Document2 pagesF4D 24Ivan WongNo ratings yet
- Cell Signaling Webquest: Part 1: Dropping SignalsDocument3 pagesCell Signaling Webquest: Part 1: Dropping Signalsa60ONo ratings yet
- Test Bank For Mechanical Ventilation 7th Edition J M CairoDocument11 pagesTest Bank For Mechanical Ventilation 7th Edition J M CairoJohnCampbellyacer100% (27)
- MARA TRIAL 2013 BIOLOGY PAPER 1 ANSWER KEYDocument43 pagesMARA TRIAL 2013 BIOLOGY PAPER 1 ANSWER KEYAhmad Imran Idzqandar100% (2)
- Basic Science and Sructure of Skin MCQsDocument92 pagesBasic Science and Sructure of Skin MCQsDr.Tawheed88% (16)
- Medical Funnotes VolumeDocument108 pagesMedical Funnotes VolumeChinNo ratings yet
- Unit 1 The Integumentary SystemDocument3 pagesUnit 1 The Integumentary SystemSharva BhasinNo ratings yet
- Chemical Reaction NotesDocument2 pagesChemical Reaction NotesalchriwNo ratings yet
- Respiratory Muscle Ultrasonography: Methodology, Basic and Advanced Principles and Clinical Applications in ICU and ED Patients-A Narrative ReviewDocument12 pagesRespiratory Muscle Ultrasonography: Methodology, Basic and Advanced Principles and Clinical Applications in ICU and ED Patients-A Narrative Reviewalejandro RodriguezNo ratings yet
- Chem 205 Lecture 5: Nucleotides and Nucleic Acids (Derived From Voet, 2011)Document37 pagesChem 205 Lecture 5: Nucleotides and Nucleic Acids (Derived From Voet, 2011)Rab BaloloyNo ratings yet
- Preparing Blood ComponentsDocument9 pagesPreparing Blood ComponentsaksinuNo ratings yet
- Nutrition Folio (Basal Metabolic Rate)Document15 pagesNutrition Folio (Basal Metabolic Rate)Nursakinah NajwahNo ratings yet
- Kantor Cabang: BANJARMASIN - 1701 FKTP: Kertak Hanyar - 17040601Document6 pagesKantor Cabang: BANJARMASIN - 1701 FKTP: Kertak Hanyar - 17040601Ic-tika Siee ChuabbieNo ratings yet
- Ineffective Airway ClearanceDocument1 pageIneffective Airway ClearanceFreisanChenMandumotanNo ratings yet
- Reaching and Being ReachedDocument18 pagesReaching and Being ReachedJoão Vitor Moreira Maia100% (1)
- Psychosomatic DisordersDocument79 pagesPsychosomatic DisordersMONIKANo ratings yet
- Pathological Postpartum Breast Engorgement Prediction, Prevention, and ResolutionDocument6 pagesPathological Postpartum Breast Engorgement Prediction, Prevention, and ResolutionHENINo ratings yet
- Alaryngeal SpeechDocument1 pageAlaryngeal Speechvee propagandaNo ratings yet
- CK-MB production method reportDocument1 pageCK-MB production method reportOkura JoshuaNo ratings yet
- FloTrac Sensor Clinical UtilityDocument21 pagesFloTrac Sensor Clinical UtilityAnestesia 2017 UDECNo ratings yet
- ECG Localization of Culprit Artery in Acute Myocardial InfarctionDocument104 pagesECG Localization of Culprit Artery in Acute Myocardial Infarctionginaul100% (1)
- Biological Level of Analysis Research GuideDocument45 pagesBiological Level of Analysis Research GuidePhiline Everts100% (2)
- Postpartum HemorrhageDocument25 pagesPostpartum HemorrhageaKmaL67% (3)
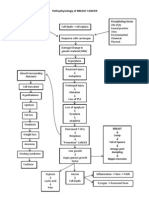
- Pathophysiology of BREAST CANCERDocument1 pagePathophysiology of BREAST CANCERAlinor Abubacar100% (6)
- Kidney PowerPoint Presentation TemplateDocument14 pagesKidney PowerPoint Presentation TemplateMedicinePPT100% (1)
- Properties of Skeletal MuscleDocument21 pagesProperties of Skeletal Musclenirilib76% (17)
- General Biology 2 Organs and Organ System: APRIL 13, 2021Document6 pagesGeneral Biology 2 Organs and Organ System: APRIL 13, 2021Patrick VerroyaNo ratings yet
- Delhi Public School Bangalore North Science Assignment KeyDocument3 pagesDelhi Public School Bangalore North Science Assignment KeyJanaki KrishnanNo ratings yet