Professional Documents
Culture Documents
Functional Group Analysis
Uploaded by
Pharmacist970 ratings0% found this document useful (0 votes)
97 views2 pagesFunctional Group Analysis
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentFunctional Group Analysis
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
97 views2 pagesFunctional Group Analysis
Uploaded by
Pharmacist97Functional Group Analysis
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Functional Group Analysis:
Hydroxyl group (-OH)
Alcohol
Phenolic
X gm of alcohol contain 17 gm of hydroxyl group
3 methods
a. Acetylation
b. Phthalation
c. Oxidation with periodates (periodic acid for polyhydroxy compounds)
a. Acetylation: R(OH)n where n is no. of hydroxyl group.
R(OH)n + (CH3CO)2O → R-O-COCH3 (Ester) + n CH3COOH (acetic acid)
Acetic acid is titrated against standard NaOH
Pyridine is used as neutral solvent due to following factors:
Inactive towards the reagent
Remove the acid produced by salt formation
Serves as a catalyst
R-OH + (CH3CO)2O → R-O-COCH3 + (C55NH)+ (CH3COO)-
(pyridinium acetate)
Acetylation reagent:
Acetic anhydride + pyridine
1 part 3 part
Preserved with a guard tube containing Na-CaCl2
NaOH solution is alcoholic.
Procedure:
(Material) + Acetic anhydride + few drops of pyridine → Reflux for 45 min + water
(converts excess of Ac2O to Acetic acid). Titrate with std. NaOH. Take one blank
Mixed Indicator:
0.1% cresol red + 0.1% thymol blue Neutralised with NaOH
1 part 3 part
Color change: pH 9.8
Calculations:
W = wt (g) of sample
V1= Vol. (ml) of NaOH ( Blank)
V2= Vol. (ml) of NaOH ( sample)
N= Normality of NaOH solution
% of hydroxyl gr : {(V1-V2) x N} / 100 x (17/ w) x 100
1000 ml of N NaOH ≡ 1-Oh group
1000 ml of N NaOH ≡ 17 gm ,, ,,
(V1-V2) 1000 ml of N NaOH ≡ {(V1-V2) x 17 x N} / 1000 gm of –OH gr.
Now, w gm contain {(V1-V2) x 17 x N} / 1000
100 gm contains (V1-V2) x 17 x N x 100
……………………………………….
1000 x w
Use of Blank Titration:
Blank gives absolute concentration of reagent. Unavailable losses of the chemicals
by glass or cork etc. will be compensated. The chemical loss will be identical.
You might also like
- Mass Spectrometry Dry Lab CHM 1021Document7 pagesMass Spectrometry Dry Lab CHM 1021himaNo ratings yet
- 3820 Lecture Chapter - 3 - Part1 - 2004 PDFDocument15 pages3820 Lecture Chapter - 3 - Part1 - 2004 PDFPhuongUblMyNo ratings yet
- ? ? Guidelines For Drinking-Water Quality? ?Document29 pages? ? Guidelines For Drinking-Water Quality? ?IRSHAD AHMED GULAMMOHIYODDIN ABBAS SHAIKHNo ratings yet
- HACH Chloride by Thiocyanate-Method No.8113-DOC316.53.01017 - Ed7Document6 pagesHACH Chloride by Thiocyanate-Method No.8113-DOC316.53.01017 - Ed7Balas43No ratings yet
- Chromatography - The Most Versatile Method of Chemical AnalysisDocument438 pagesChromatography - The Most Versatile Method of Chemical AnalysisdnabrasilNo ratings yet
- Identification of Organic and Inorganic Compounds by SpectrosDocument79 pagesIdentification of Organic and Inorganic Compounds by SpectrosAin SkNo ratings yet
- Pharmaceutical Impurities: A ReviewDocument8 pagesPharmaceutical Impurities: A ReviewparasNo ratings yet
- HPLC ColumnDocument36 pagesHPLC ColumnanakdamitNo ratings yet
- LC by DesingDocument12 pagesLC by DesingsudermanfitoNo ratings yet
- Mass Spectrometry 1Document10 pagesMass Spectrometry 1saymaNo ratings yet
- Precipitation Titration 2015Document22 pagesPrecipitation Titration 2015MaulidinaNo ratings yet
- FDA Warning LetterDocument10 pagesFDA Warning LetterKwabena Agyeman100% (1)
- Conductometric MeasurementsDocument34 pagesConductometric Measurementsmonika vermaNo ratings yet
- Uplc 2015Document26 pagesUplc 2015Hanumant ChavanNo ratings yet
- Assay of Sodium BenzoateDocument6 pagesAssay of Sodium BenzoateMeziane BouktitNo ratings yet
- HydrolysisH PDFDocument12 pagesHydrolysisH PDFEuwan Tyrone PriasNo ratings yet
- Isotopic Dilution MethodDocument3 pagesIsotopic Dilution MethodZafar IqbalNo ratings yet
- Note Guidance Manufacture Finished Dosage Form enDocument7 pagesNote Guidance Manufacture Finished Dosage Form enseshadriNo ratings yet
- Application of Column Chromatography in PharmacyDocument5 pagesApplication of Column Chromatography in PharmacyJay Shah100% (2)
- Colorimeter PDFDocument9 pagesColorimeter PDFEnrique VidalNo ratings yet
- Experiment 6: Determination of Alkali Content in Antacid Tablet Using HCLDocument6 pagesExperiment 6: Determination of Alkali Content in Antacid Tablet Using HCLVivek SehgalNo ratings yet
- E013721823 PDFDocument6 pagesE013721823 PDFAbeiasaNo ratings yet
- Chapter 4 Statistical Quality Kontrol: Statistical Process Control at Kurt ManufacturingDocument44 pagesChapter 4 Statistical Quality Kontrol: Statistical Process Control at Kurt ManufacturingAyush Mishra100% (1)
- Alkalinity, Salinity, Dissolved Oxygen, Electrical Conductivity Etc. in An Aquatic System, TheseDocument8 pagesAlkalinity, Salinity, Dissolved Oxygen, Electrical Conductivity Etc. in An Aquatic System, TheseElaiza M. PontriasNo ratings yet
- Review Impurezas PDFDocument7 pagesReview Impurezas PDFGeral RodriguezNo ratings yet
- HPLCDocument12 pagesHPLCkushalNo ratings yet
- Measuring PH CorrectlyDocument33 pagesMeasuring PH CorrectlyAnonymous Z7Lx7q0RzNo ratings yet
- Review Hyphenation in Sample Preparation: Advancement From The Micro To The Nano WorldDocument14 pagesReview Hyphenation in Sample Preparation: Advancement From The Micro To The Nano WorldNoorfatimah YahayaNo ratings yet
- Iron Experiment XWDocument4 pagesIron Experiment XWpathisharmaNo ratings yet
- BKC 80Document2 pagesBKC 80jawaidchemicalsNo ratings yet
- OrganicCarbonTotalDirectTNT DOC316.53.01093Document8 pagesOrganicCarbonTotalDirectTNT DOC316.53.01093yocam2No ratings yet
- Water PDFDocument250 pagesWater PDFkelvinNo ratings yet
- Critical Reviews in Analytical Chemistry 431782232013Document47 pagesCritical Reviews in Analytical Chemistry 431782232013Jairo William Vergara DelbastosNo ratings yet
- Potentiometric Titration of An Acid MixtureDocument9 pagesPotentiometric Titration of An Acid MixtureRuth Danielle GasconNo ratings yet
- Precipitation ChapterDocument12 pagesPrecipitation ChapterMaricica Gorceag50% (2)
- Eng - Chemsitry LabmanualDocument41 pagesEng - Chemsitry Labmanualengineeringchemistry100% (1)
- Common Ion EffectDocument6 pagesCommon Ion Effectruchi_rohilla9603No ratings yet
- Course Work For PHD in Chemistry From VtuDocument71 pagesCourse Work For PHD in Chemistry From Vtufarooq_bagbanNo ratings yet
- Titration (Chemistry Experiment Report)Document7 pagesTitration (Chemistry Experiment Report)JasgeoNo ratings yet
- Lec8 Chrom3 HPLCDocument14 pagesLec8 Chrom3 HPLCAnonymous Jlq5r8W1No ratings yet
- Derivation of Lambert-Beer - LawDocument8 pagesDerivation of Lambert-Beer - LawB1605 SAKSHAM MISHRANo ratings yet
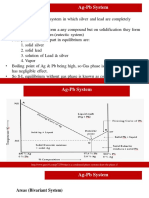
- Two Component System (Pb-Ag) and (ZN-MG) PDFDocument12 pagesTwo Component System (Pb-Ag) and (ZN-MG) PDFManiNo ratings yet
- PolarographyDocument303 pagesPolarographyHERNANDEZ1010100% (1)
- The Role of Organic Reagents PDFDocument16 pagesThe Role of Organic Reagents PDFLUIS XVNo ratings yet
- Sensitive Determination of Glutaraldehyde in Environmental Water by Derivatization and Gas Chromatography-Mass SpectrometryDocument8 pagesSensitive Determination of Glutaraldehyde in Environmental Water by Derivatization and Gas Chromatography-Mass SpectrometryAmm MarakataNo ratings yet
- 10.functional Group AnalysisDocument7 pages10.functional Group Analysisstudent_4_evaNo ratings yet
- Webinar CEP 2.0 May 2023Document51 pagesWebinar CEP 2.0 May 2023Julia ShulgaNo ratings yet
- MT ElectrodsDocument28 pagesMT ElectrodsLuisa'fer SaavedraNo ratings yet
- M.tech Environmental EngineeringDocument20 pagesM.tech Environmental Engineeringpallavi chaudharyNo ratings yet
- Gravimetric Analysis Very GoodDocument24 pagesGravimetric Analysis Very Gooddhungelsubhash8154No ratings yet
- 10 Chemistry Annual Report 2016-17Document56 pages10 Chemistry Annual Report 2016-17Hussein KyhoieshNo ratings yet
- Hypheanted Techniques in GCDocument25 pagesHypheanted Techniques in GCSalazar Robles GabrielNo ratings yet
- IndicatorsDocument6 pagesIndicatorsRajeev GangwarNo ratings yet
- BE262 Practical Microbiology and Genetics Lab Manual 2020 PDFDocument44 pagesBE262 Practical Microbiology and Genetics Lab Manual 2020 PDFsarath6142No ratings yet
- Anal Chem 3 - Test 1-2016Document4 pagesAnal Chem 3 - Test 1-2016Buhle BuhleNo ratings yet
- GC AASGasChromatography AtomicAbsorptionspectrometry Advancedspectralanalysis MPHARMACYMPCTDocument9 pagesGC AASGasChromatography AtomicAbsorptionspectrometry Advancedspectralanalysis MPHARMACYMPCTSubhasish DashNo ratings yet
- Basic Analytical Chemistry TrainingDocument23 pagesBasic Analytical Chemistry TrainingFidaa JaafrahNo ratings yet
- Assay of AspirinDocument10 pagesAssay of Aspirindavin otooleNo ratings yet