Professional Documents
Culture Documents
T-S R718
Uploaded by
Islander0 ratings0% found this document useful (0 votes)
261 views1 pageOriginal Title
T-s R718
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
261 views1 pageT-S R718
Uploaded by
IslanderCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
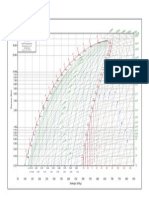
500
250
100
50
25
10
5.0
1.0 0.50 0.25 0.10 0.0500.025 0.010
2.5
1.0
0.50
10 5.0 2.5
0.25
25
0.10
50
0.050
100
0.025
250
50
DTU, Department of Energy Engineering
h in [kJ/kg]. v in [m^3/kg]. p in [Bar]
M.J. Skovrup & H.J.H Knudsen. 11-08-19
0.010
380.00
300
R718 Ref :W.C.Reynolds: Thermodynamic properties in SI
0 .0 0
400.00
h = 3200
360.00
340.00
320.00
100
300.00
280.00
50
h = 3000
240.00
25
220.00
v=
0
v=.0050
0.0
10
Temperature [C]
260.00
200.00
v=
180.00
25
0.0
160.00
.0
=0
10
50
v=
0
0.1
140.00
.2
v= 0
120.00
2.5
1.0
.50
v= 0
v= 1.0
100.00
80.00
h = 2800
5.0
0.50
0.25
v= 2.5
60.00
v= 5.0
40.00
v= 10
v= 25
20.00
0.00
x = 0.10
0.20
h = 200
-1200
-600
600
0.30
400
1200
0.40
600
1800
2400
800
3000
h = 2600
0.10
0.50
1000
3600
0.050
0.025
v= 50
0.60
1200
1400
0.010
0.70
1600
4200 4800 5400 6000
Entropy [J/(kg K)]
0.80
1800
6600
2000
7200
0.90
2200
7800
8400
2400
9000
9600 10200 10800 11400
You might also like
- Solutions Manual For Thermodynamics and Chemistry: Howard DevoeDocument110 pagesSolutions Manual For Thermodynamics and Chemistry: Howard DevoeAshna GautamNo ratings yet
- DTU Data Chart Propane PropertiesDocument1 pageDTU Data Chart Propane PropertiesNur Abdillah Siddiq100% (2)
- Advanced Heat TransferDocument17 pagesAdvanced Heat TransferDiego Serrate100% (1)
- R600a R1150Document4 pagesR600a R1150elpancasero77No ratings yet
- Friction Stir Welding of High Strength 7XXX Aluminum AlloysFrom EverandFriction Stir Welding of High Strength 7XXX Aluminum AlloysNo ratings yet
- Siemens Building Technologies: HVAC ProductsDocument299 pagesSiemens Building Technologies: HVAC Productscamus1125No ratings yet
- Dimensions and cross-sections of round copper conductorsDocument1 pageDimensions and cross-sections of round copper conductorsCipri RengleNo ratings yet
- R 134 ADocument1 pageR 134 AMarcella VitóriaNo ratings yet
- Refrigeration Graphic (Coolpack)Document27 pagesRefrigeration Graphic (Coolpack)Humaam DzulhilmiNo ratings yet
- R134a Refrigerant Property DataDocument1 pageR134a Refrigerant Property DatanotasdelingenieroNo ratings yet
- Heat Transfer EngineeringDocument14 pagesHeat Transfer EngineeringAhmedAdrarNo ratings yet
- Diagram R12Document1 pageDiagram R12Taufiq Nur75% (4)
- 01 Engineering Flow and Heat ExchangeDocument409 pages01 Engineering Flow and Heat ExchangeEdna Lisdeth Viveros NavaNo ratings yet
- Log (P) - H Diagram R134aDocument1 pageLog (P) - H Diagram R134avitorfguerra0% (1)
- AMMONIA and R134a (Satd and SH) (English and SI)Document24 pagesAMMONIA and R134a (Satd and SH) (English and SI)MinjdeDios0% (1)
- Thermotables Part16Document1 pageThermotables Part16Alberto GutierrezNo ratings yet
- Curs 0 Introducere in MatlabDocument73 pagesCurs 0 Introducere in MatlabCorinaPîrvuNo ratings yet
- Matlab ExerciseDocument8 pagesMatlab ExerciseabvibNo ratings yet
- ENSC 461 Tutorial, Week#4 - IC EnginesDocument8 pagesENSC 461 Tutorial, Week#4 - IC Enginesandres179No ratings yet
- 5 Controlled Rectifier DC Drives-Closed Loop - PpsDocument41 pages5 Controlled Rectifier DC Drives-Closed Loop - PpsRanjan KumarNo ratings yet
- ES311 Assignment & Tutorial 6Document7 pagesES311 Assignment & Tutorial 6HemanthSurya0% (1)
- Thermo Calc Console ExamplesDocument523 pagesThermo Calc Console ExamplesKarthi KeyanNo ratings yet
- Runge Kutta 4 Order - With - Solution PDFDocument4 pagesRunge Kutta 4 Order - With - Solution PDFhuma razaNo ratings yet
- Install Ubuntu Mpich WRF 4.4 Chem KPP EngDocument30 pagesInstall Ubuntu Mpich WRF 4.4 Chem KPP EngAlci DgNo ratings yet
- (Unit Operations Laboratory-2) : Name: Siraj Ali Aldeeb ID: 3214118Document11 pages(Unit Operations Laboratory-2) : Name: Siraj Ali Aldeeb ID: 3214118Siraj AL sharifNo ratings yet
- Performance Evaluation of A Air Conditioner According To Different Test Standards PDFDocument9 pagesPerformance Evaluation of A Air Conditioner According To Different Test Standards PDFIAEME PublicationNo ratings yet
- Middle East Technical University Mechanical Engineering Department ME 485 CFD With Finite Volume Method Spring 2017 (Dr. Sert)Document34 pagesMiddle East Technical University Mechanical Engineering Department ME 485 CFD With Finite Volume Method Spring 2017 (Dr. Sert)Kwanchai Choicharoen100% (1)
- HW Solution 12 FBDDocument5 pagesHW Solution 12 FBDMike DePhillipsNo ratings yet
- Chapter 5 SolutionDocument25 pagesChapter 5 SolutionNaira Classified0% (1)
- Lab Report For Ec3 Experiment: Rc-Circuits V-2 (A) For This Part We Performed The Experiment According To The Directions, Using A 30VDocument7 pagesLab Report For Ec3 Experiment: Rc-Circuits V-2 (A) For This Part We Performed The Experiment According To The Directions, Using A 30VnelimariNo ratings yet
- Spin Waves and Magnons Unit 20Document12 pagesSpin Waves and Magnons Unit 20Martin ChuNo ratings yet
- Merkezkaç Pompa Tasarımı - Temel BoyutlandırmaDocument9 pagesMerkezkaç Pompa Tasarımı - Temel BoyutlandırmaSerdarNo ratings yet
- TP Turbomachine1 Pelton Wheel Lab SheetDocument7 pagesTP Turbomachine1 Pelton Wheel Lab SheetAbdelwahab.gfNo ratings yet
- Technical Manual: Uv Photometric Ozone AnalyzerDocument118 pagesTechnical Manual: Uv Photometric Ozone AnalyzerYean-San LongNo ratings yet
- Tutorial - 6 - EntropyDocument7 pagesTutorial - 6 - EntropyanotherdeobiNo ratings yet
- Tablica 1-Iz Norme ASTM D2270-Racunanje VIDocument1 pageTablica 1-Iz Norme ASTM D2270-Racunanje VIantoNo ratings yet
- Check shear velocity and y+ valuesDocument4 pagesCheck shear velocity and y+ valueskakadeabhiNo ratings yet
- Mems Sensors For Biomedical ApplicationsDocument28 pagesMems Sensors For Biomedical ApplicationsMuraleetharan BoopathiNo ratings yet
- σ σ xxσ σ σ σ σ τ τ τ σ σ σ σ σ τ σ τ σ τ τ τ σ xx+σ σ σ: xx yy zz xy yz zxDocument4 pagesσ σ xxσ σ σ σ σ τ τ τ σ σ σ σ σ τ σ τ σ τ τ τ σ xx+σ σ σ: xx yy zz xy yz zxIrfan HaiderNo ratings yet
- Solar System: PH1800 Pro SeriesDocument2 pagesSolar System: PH1800 Pro SeriesOmar FarhatNo ratings yet
- Mece307 - Sertaç ŞimşekDocument18 pagesMece307 - Sertaç ŞimşekErtürk ErdiNo ratings yet
- 16.333 Lecture #13 Aircraft Longitudinal AutopilotsDocument32 pages16.333 Lecture #13 Aircraft Longitudinal AutopilotsArief HadiyantoNo ratings yet
- ME 363 - Fluid MechanicsDocument5 pagesME 363 - Fluid MechanicsMostafa Mohamed0% (1)
- HW4 PDFDocument11 pagesHW4 PDFJonathan MorNo ratings yet
- Optimization of CIGS Solar Cell Parameters Through SimulationDocument23 pagesOptimization of CIGS Solar Cell Parameters Through SimulationDeepak BaghelNo ratings yet
- Fundamentos Da Termodinamica Van-Wylen Exercicios Resolvidos - (Somente Exercicios para P2)Document14 pagesFundamentos Da Termodinamica Van-Wylen Exercicios Resolvidos - (Somente Exercicios para P2)Leonardo Silveira50% (2)
- Tutorial Sheet Statistical PhysicsDocument2 pagesTutorial Sheet Statistical PhysicsDIVYANSH BAJPAINo ratings yet
- Designing a Regulated Power Supply CircuitDocument19 pagesDesigning a Regulated Power Supply CircuitejalzzNo ratings yet
- Fluent-Intro 14.5 WS02 Discrete Phase PDFDocument32 pagesFluent-Intro 14.5 WS02 Discrete Phase PDFHaider AliNo ratings yet
- Instruction Manual: KI 120 Slotted Link ApparatusDocument5 pagesInstruction Manual: KI 120 Slotted Link Apparatuss_nimalanNo ratings yet
- Two-degree-of-freedom mass-damper-spring system linear dynamicsDocument2 pagesTwo-degree-of-freedom mass-damper-spring system linear dynamicsReinaldy MaslimNo ratings yet
- Parametric Study of A Dog Clutch Used in A Transfer Case For TrucksDocument46 pagesParametric Study of A Dog Clutch Used in A Transfer Case For TrucksCesar VasquesNo ratings yet
- Mathematics 15Document3 pagesMathematics 15kapindoNo ratings yet
- Specifications Concerning The Environment of Electrical and Electronic Equipment Climatic and Chemical CharacteristicsDocument61 pagesSpecifications Concerning The Environment of Electrical and Electronic Equipment Climatic and Chemical CharacteristicsGT-LUCAS BARCINo ratings yet
- R717Document1 pageR717patrickcamiloNo ratings yet
- DTU, Department of Energy Engineering Energy Systems, Refrigeration S in (KJ/ (KG K) ) - V in (M 3/kg) - T in (ºC) M.J. Skovrup & H.J.H Knudsen. 10-09-24 Ref:Peng-Robinson-Stryjek-Vera EquationDocument1 pageDTU, Department of Energy Engineering Energy Systems, Refrigeration S in (KJ/ (KG K) ) - V in (M 3/kg) - T in (ºC) M.J. Skovrup & H.J.H Knudsen. 10-09-24 Ref:Peng-Robinson-Stryjek-Vera EquationJorge Navarro DíazNo ratings yet
- Dtu, Department of Energy Engineering S in (KJ/ (KG K) ) - V in (M 3/Kg) - T in (ºc) M.J. Skovrup & H.J.H Knudsen. 13-10-02 Ref:Dupont Suva Ac9000Document1 pageDtu, Department of Energy Engineering S in (KJ/ (KG K) ) - V in (M 3/Kg) - T in (ºc) M.J. Skovrup & H.J.H Knudsen. 13-10-02 Ref:Dupont Suva Ac9000ciperu55No ratings yet
- Diagramma R404ADocument1 pageDiagramma R404AWilliam SantosNo ratings yet
- R404ADocument1 pageR404AFabian RomeoNo ratings yet
- Electricity in Fish Research and Management: Theory and PracticeFrom EverandElectricity in Fish Research and Management: Theory and PracticeNo ratings yet