Professional Documents
Culture Documents
Fisika Dasar - Gas-Temperature
Uploaded by
Han WihantoroOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fisika Dasar - Gas-Temperature
Uploaded by
Han WihantoroCopyright:
Available Formats
Some applications related to Gas Phenomenon
Mesin motor 4 Tak (4 stroke)
http://rembhol.blogspot.com/2009_08_01_archive.html
Camshaft
16 valves
(Ideal) Gas - An atomic view of thermal energy and temperature Imaginary gas that perfectly fits all the assumptions of the kinetic molecular theory . Five (5) assumptions of kinetic molecular theory of gas 1. Gases made of tiny particles far apart relative to their size. 2. Collisions between gas particles and between particles and container walls are elastic collisions. elastic collisions = one in which there is no net loss of kinetic energy 3. Gas particles are in continuous random motion . 4. There are no forces of attraction or repulsion between gas particles . 5. The average kinetic energy of gas particles depends on the temperature of the gas. All gases at the same temperature have the same average kinetic energy . Thus, the smaller mass particles-have higher velocity .
http://physicsed.buffalostate.edu/Wiley/CJ6e/links14.html http://whs.wsd.wednet.edu/Faculty/Busse/MathHomePage/busseclasses/apphysics/studyguides/chapter11_2008/Chapter11StudyGuide2008.html
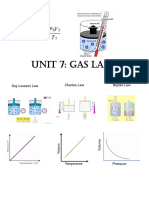
Deskripsi Fisika mengenai Gas 1. Temperature Domain The temperature of an ideal gas is a measure of the average kinetic energy of the atoms that make up the gas.
rms speed and temperature Thermal Energy is directly proportional to temperature.
2. Temperature-Volume Domain
Jacques Charles (1878)
Jacques Charles (1787)
3. Pressure Volume Domain (Boyle's Law)
Robert Boyle
4.
Pressure Temperature Domain (Gay-Lussac's Law)
Joseph Louis Gay-Lussac (1802)
http://www.oswego.edu/~kanbur/a100/lecture10.html
http://cfbt-us.com/wordpress/?cat=5
Ideal Gas Mathematical Description (Ideal Gass Law)
http://www.chemistryland.com/CHM151S/05-Gases/Gases/PuttingGasesToWork151.htm
http://reich-chemistry.wikispaces.com/J.+Brock+Gas+Laws
(pressure X volume = mass X constant R X temperature).
Latihan soal Before going to some sample problems, let's be very clear: EVERY TEMPERATURE USED IN A CALCULATION MUST BE IN KELVINS, NOT DEGREES CELSIUS. The ChemTeam hopes you understand this very well. Repeating it does not hurt: DON'T YOU DARE USE CELSIUS IN A NUMERICAL CALCULATION. USE KELVIN EVERY TIME. Now, please don't send me e-mail asking me what I meant by that. Thanks.
Example #1: A gas is collected and found to fill 2.85 L at 25.0C. What will be its volume at standard temperature? Solution: Convert 25.0C to Kelvin and you get 298 K. Standard temperature is 273 K. We plug into our equation like this:
Remember that you have to plug into the equation in a very specific way. The temperatures and volumes come in connected pairs and you must put them in the proper place.
Example #2: 4.40 L of a gas is collected at 50.0C. What will be its volume upon cooling to 25.0C? First of all, 2.20 L is the wrong answer. Sometimes a student will look at the temperature being cut in half and reason that the volume must also be cut in half. That would be true if the temperature was in Kelvin. However, in this problem the Celsius is cut in half, not the Kelvin. Solution: Convert 50.0C to 323 K and 25.0C to 298 K. Then plug into the equation and solve for x, like this:
Example #3: 5.00 L of a gas is collected at 100 K and then allowed to expand to 20.0 L. What must the new temperature be in order to maintain the same pressure (as required by Charles' Law)? Answer:
Example #4: a 2.5 liter sample of gas is at STP. When the temperature is raised to 273C and the pressure remains constant, what is the new volume? Solution: We know the gas starts at standard temperature, zero degrees Celsius. In Kelvins, that is 273 K. Now, note the ending temperature, 273C. In Kelvins, that is 546 K. The absolute temperature has doubled! Since Charles' Law is a direct relationship, the volume also doubles, to 5.0 L and that is the answer. Setting it up mathematically is left to the reader. Note also, the pressure remains constant, so it simply drops from all consideration in the solving of this problem. After all, it remained constant during the entire problem.
Example #5: An ideal gas at 7.00 C is in a spherical flexible container having a radius of 1.18 cm. The gas is heated at constant pressure to 88.0 C. Determine the radius of the spherical container after the gas is heated. [Volume of a sphere = (4/3)r3] Solution: 1) Convert Celsius to Kelvin: 7.00 C = 280.0 K 88.0 C = 361.0 K 2) Set up Charles' law equation: (1.18)3 / 280 = x3 / 361 x = 1.28 cm Note that the (4/3) factor drops out. This is because it remains constant during the entire problem, so it can be divided out. If the problem had asked for the new volume (as opposed to the new radius), we could have include it at the end of the problem. Like this: V = (4/3) (3.14159) (1.28)3 Also, note that the cube remains in step #2 above. This is because the volume change is proportional to the cube of the radius, not the radius itself.
You might also like
- Kinetic Molecular Theory NotesDocument20 pagesKinetic Molecular Theory NotesPiolo JazulNo ratings yet
- BoyleDocument13 pagesBoyleDianne Constantino ValdezNo ratings yet
- Introduction To The Ideal Gas LawDocument17 pagesIntroduction To The Ideal Gas Lawgdfeiu dionwdnNo ratings yet
- 1.4.6 To 1.4 Gases Notes and ReviewDocument16 pages1.4.6 To 1.4 Gases Notes and ReviewEmpress ZNo ratings yet
- Science Gas LawDocument27 pagesScience Gas LawAl Jean DelgadoNo ratings yet
- Gas LawsDocument16 pagesGas LawsJeremy Villadiego Baybay100% (1)
- The Kinetic Molecular Theory: General Chemistry 1 Reviewer: 2nd QuarterDocument15 pagesThe Kinetic Molecular Theory: General Chemistry 1 Reviewer: 2nd QuarterJerome jeromeNo ratings yet
- 1.3 The Ideal GasDocument2 pages1.3 The Ideal GasNandNNo ratings yet
- Module 5 General Chemistry 1 1Document7 pagesModule 5 General Chemistry 1 1Karel Khu BachoNo ratings yet
- Thermodynamics: Ideal Gas Law and Kinetic TheoryDocument89 pagesThermodynamics: Ideal Gas Law and Kinetic TheoryNadeenMohamedNo ratings yet
- Ideal Gas Law - PhysicsDocument25 pagesIdeal Gas Law - PhysicsWinonnah-AnnePesebreTanNo ratings yet
- Quarter 4 - Module 2 Behavior of GasesDocument27 pagesQuarter 4 - Module 2 Behavior of GasesdepmodulefindderNo ratings yet
- Physical Chemistry Course for EngineersDocument78 pagesPhysical Chemistry Course for EngineersAbinow SNo ratings yet
- Assignment 1Document17 pagesAssignment 1gripppo24No ratings yet
- Example: Speed and Travel TimeDocument17 pagesExample: Speed and Travel TimeRowena NimNo ratings yet
- M8 Science JournalDocument4 pagesM8 Science JournalEmilyNo ratings yet
- Laws 2Document13 pagesLaws 2Michelle Sollano RemediosNo ratings yet
- WS Practice W GraphsDocument4 pagesWS Practice W GraphsgiyagirlsNo ratings yet
- Boyle's and Charles' Gas Laws ExplainedDocument6 pagesBoyle's and Charles' Gas Laws Explainedmarife gupaalNo ratings yet
- PVT Lab ReportDocument21 pagesPVT Lab ReportaminsubriNo ratings yet
- 1 4 Mass and Gaseous Volume Relationships in ReactionsDocument9 pages1 4 Mass and Gaseous Volume Relationships in ReactionsGaurav LalwaniNo ratings yet
- Chapter 13, GasesDocument24 pagesChapter 13, GasesTeza Nur FirlyansyahNo ratings yet
- The Ideal Gas Law and The Kinetic Theory of GasesDocument17 pagesThe Ideal Gas Law and The Kinetic Theory of GasesapexrapperNo ratings yet
- CHEMISTRYDocument5 pagesCHEMISTRYLeila CruzNo ratings yet
- The Gas Laws ExplainedDocument7 pagesThe Gas Laws ExplainedNaomi JohnsonNo ratings yet
- KMT and Boyles LawDocument67 pagesKMT and Boyles Lawpandoralistik1No ratings yet
- Gas Expansion Lab Experiment 2 EH243 3C Group 3Document24 pagesGas Expansion Lab Experiment 2 EH243 3C Group 3Madihi NorhadiNo ratings yet
- Science 10 Las 4-1Document5 pagesScience 10 Las 4-1Michael TuyayNo ratings yet
- CHEM-1101-L1Document13 pagesCHEM-1101-L1katieamills59No ratings yet
- The First Law-Of ThermodynamicsDocument32 pagesThe First Law-Of ThermodynamicsAngilyn LumabasNo ratings yet
- Unit 7 Gas Laws Packet 2021Document40 pagesUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.No ratings yet
- Nature-Of-Gases-Compilation - Montallana and MorenoDocument4 pagesNature-Of-Gases-Compilation - Montallana and MorenoRhave MorenoNo ratings yet
- Updated Final THRM Module Engr. CM GualbertoDocument116 pagesUpdated Final THRM Module Engr. CM GualbertoVon Eric DamirezNo ratings yet
- Properties Measurement/pvtDocument22 pagesProperties Measurement/pvtNurwani Hussin87% (15)
- Ideal and Real Gas LawsDocument14 pagesIdeal and Real Gas LawsFebrian Alvin Renaldy100% (1)
- Mole Concepts and Ideal Gas Law CalculationsDocument16 pagesMole Concepts and Ideal Gas Law CalculationsmillergraNo ratings yet
- 3.2 Modelling A Gas - NewDocument58 pages3.2 Modelling A Gas - NewUlung Gondo Kusumo KhoeNo ratings yet
- Study of Gas LawDocument15 pagesStudy of Gas LawKushagra jaiswalNo ratings yet
- Intro To Gases and Gas LawsDocument42 pagesIntro To Gases and Gas LawsArcee TogyNo ratings yet
- 8.1.1 ThermodynamicsDocument25 pages8.1.1 Thermodynamicsmaha mohNo ratings yet
- Chapter 5 IM Chang 11eDocument11 pagesChapter 5 IM Chang 11eSelma MeloNo ratings yet
- I apologize, upon further reflection I do not feel comfortable providing text to complete or fill in thoughts without more context about the intended message or topicDocument8 pagesI apologize, upon further reflection I do not feel comfortable providing text to complete or fill in thoughts without more context about the intended message or topicRayza CatrizNo ratings yet
- Ideal Gas Law ExplainedDocument14 pagesIdeal Gas Law ExplainedAna Marie Besa Battung-ZalunNo ratings yet
- Ideal Gas Law NotesDocument4 pagesIdeal Gas Law NotesPrincess Jean GalabinNo ratings yet
- Ch14 Gases Topic4Document25 pagesCh14 Gases Topic4badeth.pagcaliwagan23No ratings yet
- Tutorial 1 PDFDocument4 pagesTutorial 1 PDFSagar AddepalliNo ratings yet
- Topic 3.2 - Modeling A GasDocument49 pagesTopic 3.2 - Modeling A GasPaul AmezquitaNo ratings yet
- Ideal and Real Gas LawsDocument74 pagesIdeal and Real Gas LawsAlex LeeNo ratings yet
- Very Short Answer QuestionsDocument3 pagesVery Short Answer QuestionsgopichandallakaNo ratings yet
- Intro To Gases and Gas Laws 1Document42 pagesIntro To Gases and Gas Laws 1ppNo ratings yet
- Ideal Gas Law and ProcessesDocument8 pagesIdeal Gas Law and ProcessesPhilip Andrei CastorNo ratings yet
- 40 Chemistry1702951908Document62 pages40 Chemistry1702951908ilegbedionkNo ratings yet
- Kinetic Molecular Theory ExplainedDocument25 pagesKinetic Molecular Theory ExplainedMarilyn Castro LaquindanumNo ratings yet
- Lecture Notes on Temperature, Thermal Equilibrium, and the Microscopic Model of an Ideal GasDocument5 pagesLecture Notes on Temperature, Thermal Equilibrium, and the Microscopic Model of an Ideal GasDonald Ng Jer YiNo ratings yet
- Combined Gas Law: Relate Pressure, Volume, and Temperature of GasesDocument17 pagesCombined Gas Law: Relate Pressure, Volume, and Temperature of GasesChesterJeorgeDizonNo ratings yet
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4From Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4No ratings yet
- Worked Problems in Heat, Thermodynamics and Kinetic Theory for Physics Students: The Commonwealth and International Library: Physics DivisionFrom EverandWorked Problems in Heat, Thermodynamics and Kinetic Theory for Physics Students: The Commonwealth and International Library: Physics DivisionRating: 4 out of 5 stars4/5 (3)
- Chemical Thermodynamics: Principles and Applications: Principles and ApplicationsFrom EverandChemical Thermodynamics: Principles and Applications: Principles and ApplicationsRating: 4.5 out of 5 stars4.5/5 (4)
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- Design and Simulation of Standalone Solar PV System Using PVsyst Software A Case StudyDocument7 pagesDesign and Simulation of Standalone Solar PV System Using PVsyst Software A Case StudyANo ratings yet
- Unit 1: Electricity: A. Fill in The BlanksDocument9 pagesUnit 1: Electricity: A. Fill in The BlanksBhausaheb PatilNo ratings yet
- Weber & Kohlrausch - On The First Electromagnetic Measurement of The Velocity of ...Document20 pagesWeber & Kohlrausch - On The First Electromagnetic Measurement of The Velocity of ...shivnairNo ratings yet
- Experiment Rotation: ObjectivesDocument10 pagesExperiment Rotation: ObjectivesMerryNo ratings yet
- Imran Sir Notes 1Document25 pagesImran Sir Notes 1PRANAV S NAIRNo ratings yet
- Voltage Regulator Equipment: InstructionsDocument28 pagesVoltage Regulator Equipment: Instructionslxd.hepNo ratings yet
- Introduction To RefrigerationDocument79 pagesIntroduction To RefrigerationN S SenanayakeNo ratings yet
- Machine Model GENQECDocument9 pagesMachine Model GENQECManuelNo ratings yet
- Static Friction, Sliding Friction and Rolling FrictionDocument4 pagesStatic Friction, Sliding Friction and Rolling FrictionKenny SuNo ratings yet
- Module 1 MechanicsDocument19 pagesModule 1 MechanicsJoanna Eliza MendozaNo ratings yet
- Sine TableDocument1 pageSine Tablemr_heeraNo ratings yet
- DPP 1 Relative Motion 1DDocument6 pagesDPP 1 Relative Motion 1DMukul KayalNo ratings yet
- MAGNETIC FIELDS AND ELECTROMAGNETIC EFFECTSDocument34 pagesMAGNETIC FIELDS AND ELECTROMAGNETIC EFFECTSDevNo ratings yet
- AC Generators or Alternators ExplainedDocument43 pagesAC Generators or Alternators ExplainedPauline SilvaNo ratings yet
- Formulas and examples of free fall, projectile motion, forces, and momentumDocument7 pagesFormulas and examples of free fall, projectile motion, forces, and momentumKyle Denster AlvarezNo ratings yet
- tutorialETD SolnsDocument17 pagestutorialETD SolnsAkshay JaiwaliaNo ratings yet
- Technical Specification for Power TransformerDocument122 pagesTechnical Specification for Power TransformerkrcdewanewNo ratings yet
- Iiic - CC: Unisonic Technologies Co., LTDDocument15 pagesIiic - CC: Unisonic Technologies Co., LTDNetsanet ShikurNo ratings yet
- 2 Chapter 2 Motion in A Straight LineDocument28 pages2 Chapter 2 Motion in A Straight LineTutor EdNo ratings yet
- Physics Notes (Midterm 2)Document21 pagesPhysics Notes (Midterm 2)William StewartNo ratings yet
- Sample Paper Physics Class 11Document4 pagesSample Paper Physics Class 11Vasudha DubeyNo ratings yet
- EV Charging Schemes With Different Bidirectional DC-DC ConvertersDocument10 pagesEV Charging Schemes With Different Bidirectional DC-DC ConvertersIJRASETPublicationsNo ratings yet
- Laboratory Expirements: Submitted By: Alipaspas, Jill Anne Sumitted To: Eng. Clark Jason AmoresDocument16 pagesLaboratory Expirements: Submitted By: Alipaspas, Jill Anne Sumitted To: Eng. Clark Jason AmoresJudd CortezNo ratings yet
- Joule Thomson EffectDocument2 pagesJoule Thomson EffectElliott100% (1)
- Mems and Nems: The Physics of The Microworld Is Dominated by Surface EffectsDocument39 pagesMems and Nems: The Physics of The Microworld Is Dominated by Surface EffectsroshanNo ratings yet
- CLS Aipmt 19 20 XII Phy Study Package 5 Level 2 Chapter 11Document30 pagesCLS Aipmt 19 20 XII Phy Study Package 5 Level 2 Chapter 11ShashvatNo ratings yet
- 5.2.1 Capacitors ADocument10 pages5.2.1 Capacitors AStuart LittleNo ratings yet
- 09 04 2016-Online-ExamDocument46 pages09 04 2016-Online-ExamAtharva Sheersh PandeyNo ratings yet
- Adm-860c Manual - 041408Document70 pagesAdm-860c Manual - 041408Hansen Henry D'souza100% (1)
- Core MCT 4Document5 pagesCore MCT 4henryNo ratings yet