Professional Documents
Culture Documents
Scbbyw
Uploaded by
Uchiha IkhastoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Scbbyw
Uploaded by
Uchiha IkhastoCopyright:
Available Formats
MOLECULAR STRUCTURE OF NUCLEIC ACIDS
structure has novel f@8tUIW which biological intewst. A structure for nucleic acid proposed by p8uling and Corey*. their manuscript 8vailablo to
A Structure for Deoxyribose Nucleic Acid j3 wish to suggecit 8 structure for the salt ThiR of deoxyribose nuclei0 void (D.N.A.).
8IX3of considereblc
has already been They kindly m8de
us in .8dvence of
publication. Their model conhtR of three intar- &ucturo in the most plausible tautomeric formrr twined cheins, with t.ho phosphates near lhe Abre (thet is, with the keto rather than the onol oon-
is a residue on each chain every 34 A. in the t-dim@ tion. We have assumed an 8&e of 36 between edjeccnt reaiducs in the same chain, so that the structure repeats after 10 residues on each ohain. that is, after 34 A. The dietenoo of 8 phosphorus atom tirn the flbre exis is 10 A. AE t.he phosphates erc on the outside, cations have esey 800888 to them: The structure is an open one, 8nd its w8tM Content is rather high. At lower water contents we would expect the bases to tilt so that the structure could become more comp8ct. The novel feature of the structure is the manner in which the two cheins are held %ogether by the purine and pyrimidine beses. The plenea of the ba6es are perpendicular to the fibro oxis. They are joined together in pairs, a single beee from one chain being hydrogen-bonded to a single base from the other chain, sc that the two lie side by side with identical z-co-ordinato8. One of the pair must be 8 purine and the other 8 pyrimidine for bonding to occur. The hydrogen bonds are made 8s follows : purine pcsition 1 to pyrimidine position 1 ; purine position 6 to pyrimidino position 6. If it is 8ssumed that the bases only occur in the
8x& and the bases on the outside. In our bpinion, this structure is un.tiefectory for two reasons : (1) We believe thnt the meteri which gives the X-ray diagr8ms is the s8lt. not the free noid. Without t.he ecidio hydrogen 8toms it is not clear Whet forces would hold the structure t.ogethcr, especially 88 the nega?ivcly cherged phosphates ne8r t.he 8ti will repel each otlor. (2) Some of t,ho v8n der Waals dist8nces 8ppcsr to be too smell. Another thrcc-chein structure h8s also been suggcstod by Fro&r (in t,he press). .Tn his model the phospkatcs 8~) on the out&do 8nd Yhc bases on tho inside, -link4 together by hydrogen bonds. This structure BR described is tathor ill-dcflned, nnd for this mason we shall not comment on it. We wish to put forw8rd a mdic8lly diffcrcnt structure for the eelt of deoxyribose nucleic This structure haa two acid.
of the amounta of adenine to thymine, and the ratio
Qurations) it is found that only qx&ic pairs of b8ses c8n bond together. Thes8 pairs are : ad&nine (purine) with thymine (pyrimidinc), and gu81~i.1~ (purine) with cytosine (l~yrimidine). In other words, if 8n adenine forms one member of 8 pair, on oither chain, then on these assumptions the other member must be t.hymine ; similarly for gu8nine and oytosine. The sequence .of base8 on 8 sing10 chein does not 8ppe8r.to be restricted in 8ny way. However, if only specifli~ peirs of baeea can be formed, it follows thet if the sequence of b+?ea on one oh8in in given, then tho seqtronce on the other &bin is 8utomatic8lly deterniined. . ! It has been found experimentally ~4 thrrt the ratio
c&x Waals conttbct. The previously published.X-ray data@& deoxyon riboee nucleic acid am insufilcient for a rigorous test have made the usuel chemical ARsumptions, nsmely, thet each of our structure. So far as we can tell, it is roughly chein consists of phoqhate di- compatible with the experimental do&, but it muat e&x groups joining p-D-deoxy- be regarded as unproved until it has been oheoked helical chains each coiled round the -me axis (see di8gram). We
ribofumnose r&&dues with 3 ,5 hk8gCS. The two chains (but not their b8s~) 8re rel8ted by 8 dyad perpendicular to the fibre 8XiS. Both chains follow righthanded helices, but owing to the dyad the scquencoe of the taoma in the t.wo. ohains run in opposita directions. Each Clulin loosely resembles Furberg model No. 1 ; th8t is, s* the bases 8re on the inside of thcr helix and t,he phosphet@s on the ciutside. The cor@ur8tion of the sug8r end t,he 8toms ne8r it is close to FUrberg s standard cotiguration , the sugar being roughly perpendicular to t.he aMached base. Them
Of gUeIliIle t0 CytOSilU3,8I 81W838 VW& OloSe t0 unity e for deoxyribosc nucleic 8cid. It is probably impossible to build .this etruature with a ribose sug8r in pleoo of the deoxyribcee, as the extn, oxygen atom would make too close a van
against more exact results. Some of these m given in the following communic8tiona. We wcm not (swam of t,he details of the results pmeented them when F
devkd
our structure, which resta mainly though not
This llgurc Is purely cllaprammrtic. The two ribbonm nymbollse the two pho@phatwu&w chains. and the horlmntal zoda the paim of basea boldln~ the chalm touether. The vertical lhc marka the fibre asln
ditions assumed in building it, tqether with a set of co-ordinates for the atoms, will be pubkhed elsewhere. We ew much indebt& to Dr. Jerry Doaohua for oonatsnt advice and orNoiam, orpsorally on inter8tOmiC distances. We have also been 8tim&te& 8 knOWledg@3 tb (Isarsti -turr, Of the Of experimental multi aad idaas of Dr.,T . H..F. Wilkins, Dr. R. E, Franklin and their oo-wotha at
entimly on published experimental date cad attainchemical e rguments. t It has not escaped our nkioe thet the! apeoiso pairing we h8ve poatul8ted imme&tely &eetS 8 possible copying meohaniem for the n&o m&Al. Full details of the atructum, inoff uding tlw tmn-
738 aided by s fellow&p from for Infantile Persly&.
the
NATURE
National Foundation
-April 25;1953
VOL. 171
King CdAege,London. One of ua (J. D. W.) haa b&m s
J. D. WATSON s. lx. a. Cmoa tiediResearch ~ounoil Unit for the Study of the MoIeouk Structure of Biological Systems, .$&wndiah L&oratory, Cambridge. April 2.
Nd. dad. Sci.. 28, 84 (1963). Fruberp, 8.. dds Chms. scord.. 6, 634 (1952). churpd. E.. for Mfwencu eea 55amcllbofl 9.. BMmnnall. . chum& E.. Bfahin. St Biophm da, 0. 402 (lM2). Wstt. 0. B., J. C2m. PhwioZ.. 80, 201 (1952). Aatbw,
Univ. * W~ikh. PrM# 1947). M. H. F., aad 10, 192 WMS).
.Panlint?. I,., and Cmey. B.. Nature, 17l, 346 (1958) ; Pme. U.S. it.
0.. md
W. T.. Symp. SOC. Exp. Bbl, Bamh&
1, Nucleic Acid, 66 (Camb. et Efephy8.
Ado.
J. T.. Bio&m.
You might also like
- Dna Structure and FunctionDocument12 pagesDna Structure and FunctionAliah Aziz100% (1)
- OPP 3 Study Guide Exam 3Document158 pagesOPP 3 Study Guide Exam 3Fazal DalalNo ratings yet
- Overcome Blushing PDFDocument108 pagesOvercome Blushing PDFManelsensteinerbergton50% (2)
- Understanding the Complex Structure and Function of the Human BrainDocument2 pagesUnderstanding the Complex Structure and Function of the Human Brainbubblegumlover96No ratings yet
- Evidence For 2-Chain Helix in Crystalline Structure of Sodium Deoxyribonucleate Franklin Nature 1953Document2 pagesEvidence For 2-Chain Helix in Crystalline Structure of Sodium Deoxyribonucleate Franklin Nature 1953Abhay Kumar100% (1)
- Atomic Theory Explained: Elements, Compounds and the Discovery of Subatomic ParticlesDocument8 pagesAtomic Theory Explained: Elements, Compounds and the Discovery of Subatomic ParticlesBill BiazonNo ratings yet
- Rules For Ring ClosureDocument3 pagesRules For Ring ClosurecrazychemistryNo ratings yet
- Hong Kong LolDocument18 pagesHong Kong Lolpewep2No ratings yet
- Russell, Bertrand - ABC of Atoms (Dutton, 1923) PDFDocument171 pagesRussell, Bertrand - ABC of Atoms (Dutton, 1923) PDFGerardo HerculesNo ratings yet
- What Is An AtomDocument3 pagesWhat Is An AtomDeepukavyaNo ratings yet
- BSC Nursing Syll PDFDocument164 pagesBSC Nursing Syll PDFRajalakshmi Srinivasan100% (1)
- Structure and Properties Oi Isotactic PolypropyleneDocument12 pagesStructure and Properties Oi Isotactic PolypropyleneNguyễn Mạnh TuấnNo ratings yet
- Molecular Structure of Nucleid AcidsDocument7 pagesMolecular Structure of Nucleid AcidsMonica MenaNo ratings yet
- La Estructura Del DNA. Watson Crick (1953)Document2 pagesLa Estructura Del DNA. Watson Crick (1953)Daniel Alejandro Rojas ToroNo ratings yet
- Watson and Crick 1953 PDFDocument2 pagesWatson and Crick 1953 PDFijigar007No ratings yet
- A Structure For Deoxyribose Nucleic Acid - CommentedDocument6 pagesA Structure For Deoxyribose Nucleic Acid - CommentedUalisson FerreiraNo ratings yet
- Articulo-Watson Crick PDFDocument2 pagesArticulo-Watson Crick PDFAjedrez ItineranteNo ratings yet
- 5'eutron Ies Zinc Ferrite Ani. Errite : 3ii:1:rection Stuc:5'icDocument6 pages5'eutron Ies Zinc Ferrite Ani. Errite : 3ii:1:rection Stuc:5'icBhabani Sankar SwainNo ratings yet
- Nonlinear Dynamics and Statistical Physics of DNADocument34 pagesNonlinear Dynamics and Statistical Physics of DNAFlaviana CatherineNo ratings yet
- J. D. Watson and F. H. C. Crick - Molecular Structure of Nucleic Acids: A Structure For Deoxyribose Nucleic AcidDocument3 pagesJ. D. Watson and F. H. C. Crick - Molecular Structure of Nucleic Acids: A Structure For Deoxyribose Nucleic AcidYopghm698No ratings yet
- Joanna I. Sułkowska, Piotr Sułkowski, P. Szymczak and Marek Cieplak - Tightening of Knots in ProteinsDocument4 pagesJoanna I. Sułkowska, Piotr Sułkowski, P. Szymczak and Marek Cieplak - Tightening of Knots in ProteinsKeomssNo ratings yet
- Week 14 Lecture 560B On LineDocument9 pagesWeek 14 Lecture 560B On LineTheNourishedSproutNo ratings yet
- 2013 Lect2a Physical Properties and Structure Relationship1Document98 pages2013 Lect2a Physical Properties and Structure Relationship1Beatrix Gloria TahaparyNo ratings yet
- Nucleic Acid (Organic Chemistry) : By: Septiana Saputri (1800023180)Document7 pagesNucleic Acid (Organic Chemistry) : By: Septiana Saputri (1800023180)septianasptrNo ratings yet
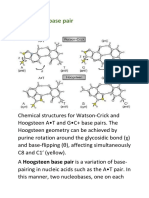
- Hoogsteen Base Pair PDFDocument7 pagesHoogsteen Base Pair PDFNikitaNo ratings yet
- Radioactivity Decay and ApplicationsDocument8 pagesRadioactivity Decay and ApplicationsdanielmahsaNo ratings yet
- Longuet-Higgins: Studies in Molecular Orbital Theory I: Resonance & Molecular Orbitals in Unsaturated HydrocarbonsDocument11 pagesLonguet-Higgins: Studies in Molecular Orbital Theory I: Resonance & Molecular Orbitals in Unsaturated HydrocarbonsvanalexbluesNo ratings yet
- Chymotrypsin MechnismDocument8 pagesChymotrypsin MechnismJoshua MckinneyNo ratings yet
- Structure of DNA by CrickDocument8 pagesStructure of DNA by CrickDr. SHIVA AITHALNo ratings yet
- Handouts Unit-IVDocument11 pagesHandouts Unit-IVmukeshNo ratings yet
- Structure of SpinelDocument14 pagesStructure of SpinelAlin DrucNo ratings yet
- Organic Chemistry MilestonesDocument9 pagesOrganic Chemistry MilestonesAj Benito MalidomNo ratings yet
- April 25, 1953 Nature 737: Molecular Structure of Nucleic Acids A Structure For Deoxyribose Nucleic AcidDocument2 pagesApril 25, 1953 Nature 737: Molecular Structure of Nucleic Acids A Structure For Deoxyribose Nucleic AcidMontaño BrandonNo ratings yet
- Nuclear DataDocument35 pagesNuclear DataOMARNo ratings yet
- April 25, 1953 Nature 737: Molecular Structure of Nucleic Acids A Structure For Deoxyribose Nucleic AcidDocument2 pagesApril 25, 1953 Nature 737: Molecular Structure of Nucleic Acids A Structure For Deoxyribose Nucleic AcidMontaño BrandonNo ratings yet
- DNA STRUCTURE AND REPLICATIONDocument21 pagesDNA STRUCTURE AND REPLICATIONDerrick kinyaNo ratings yet
- Scorpion Venom PharmacologyDocument5 pagesScorpion Venom PharmacologySutirtho MukherjiNo ratings yet
- Journal: Structure of SpinelDocument14 pagesJournal: Structure of SpinelRaul EvertonNo ratings yet
- Bonding 221217 205618Document16 pagesBonding 221217 205618Ibse ussoNo ratings yet
- Isotopes and Radioactive Tracers in BiologyDocument1 pageIsotopes and Radioactive Tracers in BiologyNathan HudsonNo ratings yet
- Structure of Cytochrome C: Protein Folding ModelsDocument6 pagesStructure of Cytochrome C: Protein Folding Modelsprashant sainiNo ratings yet
- Complementary StrandDocument20 pagesComplementary StrandJhon Mark G. HernandezNo ratings yet
- Systematics of The Spinel Structure TypeDocument23 pagesSystematics of The Spinel Structure TypeAlin DrucNo ratings yet
- Molecular Models PDFDocument12 pagesMolecular Models PDFMohsen SharifNo ratings yet
- Nature: February 21, 1953Document1 pageNature: February 21, 1953sethNo ratings yet
- Chapter 1Document11 pagesChapter 1J.K HomerNo ratings yet
- Nucleic Acid StructureDocument11 pagesNucleic Acid StructurenadirappNo ratings yet
- Covalent Bonding Theory ExplainedDocument50 pagesCovalent Bonding Theory ExplainedMinh TieuNo ratings yet
- FoldingDocument3 pagesFoldingsahoo_tpionNo ratings yet
- Some Important Topics To Get Started With The Study of Organic Pharmacy II'Document9 pagesSome Important Topics To Get Started With The Study of Organic Pharmacy II'Apurba Sarker ApuNo ratings yet
- Atomic Structure Notes Ohis State UniversityDocument4 pagesAtomic Structure Notes Ohis State Universityapi-246187169No ratings yet
- Systematics of The Spinel Structure Type: Physics I) Chemistry MiheralsDocument23 pagesSystematics of The Spinel Structure Type: Physics I) Chemistry MiheralsBassManNo ratings yet
- Jurnal KimiaDocument6 pagesJurnal KimiaImaNo ratings yet
- MatterDocument5 pagesMattercsschoonNo ratings yet
- Current Ohmos: Electric LAWDocument25 pagesCurrent Ohmos: Electric LAWMostar NNo ratings yet
- Ohm's Law and Modern Electron TheoryDocument25 pagesOhm's Law and Modern Electron TheoryMostar NNo ratings yet
- 1515564117CHE P1 M15 EtextDocument14 pages1515564117CHE P1 M15 Etextsushant mouleNo ratings yet
- Chem2161-Unit 1 PDFDocument22 pagesChem2161-Unit 1 PDFSelam 1No ratings yet
- 51a Chapter 1 2014 Copy 2Document37 pages51a Chapter 1 2014 Copy 2Efrain AnayaNo ratings yet
- Components of RNA and DNADocument26 pagesComponents of RNA and DNAKarla Javier PadinNo ratings yet
- Serine Proteases MechanismDocument6 pagesSerine Proteases MechanismCláudio NogueiraNo ratings yet
- HDTD-B-9 - Molecular Genetics IDocument25 pagesHDTD-B-9 - Molecular Genetics IMariam QaisNo ratings yet
- Olson Et Al. - 1981 - Crystal Structure and Structure-Related Properties of ZSM-5Document6 pagesOlson Et Al. - 1981 - Crystal Structure and Structure-Related Properties of ZSM-5Hari NarayananNo ratings yet
- Tetrahedron Reports on Organic Chemistry: Volume 1.1-10From EverandTetrahedron Reports on Organic Chemistry: Volume 1.1-10Derek BartonNo ratings yet
- Lichen Planus-Like Drug Eruptions Due to Beta-BlockersDocument5 pagesLichen Planus-Like Drug Eruptions Due to Beta-BlockersRudnapon AmornlaksananonNo ratings yet
- Hemorrhage and HemostasisDocument36 pagesHemorrhage and HemostasisRinor MujajNo ratings yet
- DNeasy Blood & Tissue HandbookDocument62 pagesDNeasy Blood & Tissue HandbookPeter Hong Leong CheahNo ratings yet
- CARBOHYDRATES CHEMISTRY, Lecture For 1st Year M B B S - Delivered by DR Mohammad Waseem KausarDocument22 pagesCARBOHYDRATES CHEMISTRY, Lecture For 1st Year M B B S - Delivered by DR Mohammad Waseem KausarIMDCBiochem60% (5)
- Biology Revision Essay Questions and Answers For Secondary Kusoma - Co - .KeDocument48 pagesBiology Revision Essay Questions and Answers For Secondary Kusoma - Co - .Kestevewanji0% (1)
- Guillain BarreDocument6 pagesGuillain BarreValentín PalmNo ratings yet
- Carbohydrates PDFDocument8 pagesCarbohydrates PDFWrigley PatioNo ratings yet
- Global Burden of Bronchial Asthma and Community-Based Management /TITLEDocument43 pagesGlobal Burden of Bronchial Asthma and Community-Based Management /TITLEMarius-Sorin CionteaNo ratings yet
- ESMO Epidemiology Classification and Clinical Presentation of NETs A European PerspectiveDocument39 pagesESMO Epidemiology Classification and Clinical Presentation of NETs A European PerspectiveMario MutuleanuNo ratings yet
- Tibial Plateau FractureDocument2 pagesTibial Plateau FracturecarlossantanderNo ratings yet
- The Importance of Biopharmaceutics and PharmacokineticDocument9 pagesThe Importance of Biopharmaceutics and PharmacokineticWella AfrianiNo ratings yet
- ImciDocument3 pagesImciJohn Benzon0% (1)
- Thanksgiving Sleep Questions and AnswersDocument4 pagesThanksgiving Sleep Questions and Answersapi-420563491No ratings yet
- Glucocorticoid Signaling From Molecules to Mice to ManDocument387 pagesGlucocorticoid Signaling From Molecules to Mice to ManKuldeepSinghBanaNo ratings yet
- Answer Guide For O Level Biology (5090/3) - Paper 3 Practical Test June 2001Document3 pagesAnswer Guide For O Level Biology (5090/3) - Paper 3 Practical Test June 2001MSHNo ratings yet
- Chemistry ProjectDocument15 pagesChemistry ProjectAr -JuNNo ratings yet
- Bone Marrow ExaminationDocument1 pageBone Marrow ExaminationCloudy GemsNo ratings yet
- Aiapget2018 Q&ADocument23 pagesAiapget2018 Q&ASatyam SinghNo ratings yet
- Company Most Recent Adposition (S) First Name Last Name Contact TypeDocument8 pagesCompany Most Recent Adposition (S) First Name Last Name Contact TypepaytencasNo ratings yet
- Heme Degradation and Jaundice: Molecular Biochemistry IIDocument40 pagesHeme Degradation and Jaundice: Molecular Biochemistry IInarasaiyanhariharanNo ratings yet
- MEDI203 2017 - Session 2 ExamDocument19 pagesMEDI203 2017 - Session 2 ExamAadilNo ratings yet
- A6. Karring T, Lang N, Löe H. The Role of Gingival Connective Tissue in Determining Epithelial Differentiation. J Perio Res 1975Document12 pagesA6. Karring T, Lang N, Löe H. The Role of Gingival Connective Tissue in Determining Epithelial Differentiation. J Perio Res 1975Daniel CubasNo ratings yet
- Pharmaceutical Sales Medical Devices in Orlando FL Resume Jorge Herrera SotoDocument3 pagesPharmaceutical Sales Medical Devices in Orlando FL Resume Jorge Herrera SotoJorgeHerraSotoNo ratings yet
- Vadlapudi & Naidu (2009) Evaluation of Antioxidant Potential of Selected Mangrove PlantsDocument4 pagesVadlapudi & Naidu (2009) Evaluation of Antioxidant Potential of Selected Mangrove PlantsYvonne TongNo ratings yet
- Location and External Anatomy of The KidneysDocument15 pagesLocation and External Anatomy of The KidneysKyla Malapit GarvidaNo ratings yet