Professional Documents
Culture Documents
PES Question
Uploaded by
Ding30180Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PES Question
Uploaded by
Ding30180Copyright:
Available Formats
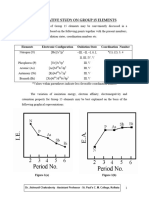
Consider the PES spectra shown below, that superimposes the simulated PES spectra for elemental boron
and elemental carbon on the same plot. NOTE: x, y and z are simply labels and do NOT refer to px, py and pz orbitals.
(a) Suggest a reason why the boron and carbon peaks have been paired together in three groups labeled x, y and z. (2)
(b) Explain why the carbon x peak is at a higher energy than the boron x peak. (2)
(c) Why is the boron z peak half the height of the carbon z peak? (1)
Question 1 continued: (d) If one were to superimpose a third PES plot on the same axes for elemental nitrogen; (i) Relative to carbons x peak, where would nitrogens x peak appear on the x-axis? Explain. (2)
(ii)
Relative to carbons z peak, what would the height of nitrogens z peak, be? Explain. (2)
(e) Identify the electrons that are associated with each peak in the boron plot. (3) (i) (ii) boron x peak boron y peak
(iii) boron z peak
You might also like
- Chapter 9 Additional Problem Answers 2022Document12 pagesChapter 9 Additional Problem Answers 2022Marta TogatoropNo ratings yet
- Solutions to Problem set on molecular orbital theory and acid-base propertiesDocument3 pagesSolutions to Problem set on molecular orbital theory and acid-base propertiesLinwen Zhang100% (1)
- 2ndqtrpracticeMT Answer KeyDocument6 pages2ndqtrpracticeMT Answer KeyMysticNo ratings yet
- Covalent Bonding GuideDocument2 pagesCovalent Bonding GuideaiNo ratings yet
- CHE 1010 Tutorial Sheet 3Document5 pagesCHE 1010 Tutorial Sheet 3Chimuka Onson MapikiNo ratings yet
- Pre-Bio 200 Chemistry Skills Quiz - Practice Questions: H O C C C H H H H H H H HDocument4 pagesPre-Bio 200 Chemistry Skills Quiz - Practice Questions: H O C C C H H H H H H H HLen Hill0% (1)
- HW1 2015Document2 pagesHW1 2015rrrrNo ratings yet
- Chapter 10Document18 pagesChapter 10Khaled NaseerNo ratings yet
- Chemistry Assignment 4 Class 11Document3 pagesChemistry Assignment 4 Class 11Nayan ShahNo ratings yet
- CHAPTER+6+Drills Tro F14Document5 pagesCHAPTER+6+Drills Tro F14PAUL ALEGRENo ratings yet
- General Chemistry I - Tutorials 6 and 7Document10 pagesGeneral Chemistry I - Tutorials 6 and 7Duc Anh NguyenNo ratings yet
- Free Study Materials for All ClassesDocument27 pagesFree Study Materials for All ClassesDivyanshu YadavNo ratings yet
- Document 5Document3 pagesDocument 54vxwdbk5x4No ratings yet
- Strprop chptr5Document31 pagesStrprop chptr5email semuaNo ratings yet
- Chemistry 102 Summary July 2ndDocument5 pagesChemistry 102 Summary July 2ndParth BhaskarNo ratings yet
- Ib Chemistry Chap 14 HLDocument1 pageIb Chemistry Chap 14 HLbiofireNo ratings yet
- Chemical Bonding and Molecular StructureDocument25 pagesChemical Bonding and Molecular Structureshah khisarwNo ratings yet
- Chemistry 4110-5110 (Fall 2010) Name - Problem Set #1 (Due Monday, Sept. 13)Document15 pagesChemistry 4110-5110 (Fall 2010) Name - Problem Set #1 (Due Monday, Sept. 13)Nicholas ThompsonNo ratings yet
- Lewis StructureDocument7 pagesLewis StructureKed LukkedNo ratings yet
- Basic Questions: Chapter 9: Covalent Bonding: OrbitalsDocument8 pagesBasic Questions: Chapter 9: Covalent Bonding: OrbitalsDema IhabNo ratings yet
- A Proof of Hessenberg'S TheoremDocument3 pagesA Proof of Hessenberg'S TheoremJacksonNo ratings yet
- Atom TestDocument1 pageAtom TestAnson Ka Kin ChanNo ratings yet
- Principles of Nuclear Physics (NPE-503) : M.Sc. (Nuclear Power Engineering)Document1 pagePrinciples of Nuclear Physics (NPE-503) : M.Sc. (Nuclear Power Engineering)Asif Khan NiaziNo ratings yet
- Questions on Fundamental Particles: Cs Cs Cs Cs β β β βDocument10 pagesQuestions on Fundamental Particles: Cs Cs Cs Cs β β β βjoeNo ratings yet
- General Chemistry I - Tutorial 5Document6 pagesGeneral Chemistry I - Tutorial 5Duc Anh NguyenNo ratings yet
- Name - Period - AP Chemistry Unit 2 WorksheetDocument4 pagesName - Period - AP Chemistry Unit 2 Worksheetburcak gecNo ratings yet
- 11 Chemistry Notes Chapter 4Document25 pages11 Chemistry Notes Chapter 4prashanthNo ratings yet
- CH105Inorg Tutorial III Qs OnlyDocument2 pagesCH105Inorg Tutorial III Qs OnlyKushNo ratings yet
- Practice Questions - Weeks 345 - 3rdedDocument5 pagesPractice Questions - Weeks 345 - 3rdedJon LevinsNo ratings yet
- 11 Chemistry Chapter 4 Assignment 1Document1 page11 Chemistry Chapter 4 Assignment 1amal gainNo ratings yet
- Pes Questions From Russ Maurer KeyDocument4 pagesPes Questions From Russ Maurer KeyMackenzie VoorheesNo ratings yet
- Chemical Bonding-1Document2 pagesChemical Bonding-1Anish KumarNo ratings yet
- Covalent Bonding, Electronegativity, and Bond Polarity (Sections 8.3 and 8.4)Document3 pagesCovalent Bonding, Electronegativity, and Bond Polarity (Sections 8.3 and 8.4)CRISTINA MUÑOZ CASTAÑONo ratings yet
- Atomic Structure 3 PDFDocument15 pagesAtomic Structure 3 PDFNashraat BukhoryNo ratings yet
- Chemical Bonding & Molecular Structure QuestionsDocument6 pagesChemical Bonding & Molecular Structure QuestionsPratapSinghMuniaNo ratings yet
- Answers To PE3 pgs1 5Document5 pagesAnswers To PE3 pgs1 5Jowel MercadoNo ratings yet
- Particle Physics WorksheetDocument6 pagesParticle Physics WorksheetPercy Carlos KasambiraNo ratings yet
- Assignment 1 CHM 102Document5 pagesAssignment 1 CHM 102yo yoNo ratings yet
- Midterm 2.1 Textbook Practice Problems (Chapter 14)Document10 pagesMidterm 2.1 Textbook Practice Problems (Chapter 14)Mar Ariana PerezNo ratings yet
- PHY3 CJanuary 2006Document1 pagePHY3 CJanuary 2006api-3726022No ratings yet
- Comparative-Study-of-Group-15-ElementsDocument20 pagesComparative-Study-of-Group-15-ElementsUmesh JogeNo ratings yet
- Chemical Bonding and Molecular StructureDocument15 pagesChemical Bonding and Molecular StructureSoham NagNo ratings yet
- Particles and Waves Course Questions and SolutionsDocument33 pagesParticles and Waves Course Questions and SolutionsDaveyNo ratings yet
- Solutions Chang Chapter 10Document22 pagesSolutions Chang Chapter 10Nathy_OlateNo ratings yet
- Organic ChemistryDocument3 pagesOrganic ChemistryMohammed AltahirNo ratings yet
- Chemical BondingDocument5 pagesChemical BondingYanti FarhanaNo ratings yet
- The Orbitals: Electron Density Relates To How Much of An Electron's Charge Is Packed Into A GivenDocument3 pagesThe Orbitals: Electron Density Relates To How Much of An Electron's Charge Is Packed Into A Givenkey_01No ratings yet
- Chemical Bonding and Molecular StructureDocument25 pagesChemical Bonding and Molecular StructureStudy TimeNo ratings yet
- ExamQuestionsTroChapters9 10 TrimmedDocument12 pagesExamQuestionsTroChapters9 10 TrimmedAli TarekNo ratings yet
- PHY3 CJanuary 2003Document1 pagePHY3 CJanuary 2003api-3726022No ratings yet
- Assignment 8 - Solutions Chem1000ADocument4 pagesAssignment 8 - Solutions Chem1000AXdyne67% (3)
- CH 26 Molecular Structure Problems Questions OnlyDocument8 pagesCH 26 Molecular Structure Problems Questions OnlyYocobSamandrewsNo ratings yet
- Revision Worksheet - Chemical Bonding and Molecular Structure-2022-23Document2 pagesRevision Worksheet - Chemical Bonding and Molecular Structure-2022-23Malolan SriramNo ratings yet
- On Menelaus' TheoremDocument6 pagesOn Menelaus' TheoremYishakNo ratings yet
- Che s4 Exam Term 1 - QP - 074533Document4 pagesChe s4 Exam Term 1 - QP - 074533nshimiyimanasamuel1983No ratings yet
- Bonding in Polyatomic Molecules: TopicsDocument44 pagesBonding in Polyatomic Molecules: TopicsAcikaNo ratings yet
- IFS Chemistry 2014Document5 pagesIFS Chemistry 2014lock stock and barrelNo ratings yet
- IE 114 Material Science and General Chemistry Recitation #3Document2 pagesIE 114 Material Science and General Chemistry Recitation #3azizieh5701No ratings yet
- Hyperbolic Functions: with Configuration Theorems and Equivalent and Equidecomposable FiguresFrom EverandHyperbolic Functions: with Configuration Theorems and Equivalent and Equidecomposable FiguresNo ratings yet