Professional Documents
Culture Documents
Fe C Diagram
Uploaded by
churanjitCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fe C Diagram
Uploaded by
churanjitCopyright:
Available Formats
134
Introduction to Basic Manufacturing Processes and Workshop Technology
iron
1 60 0 A 1 50 0
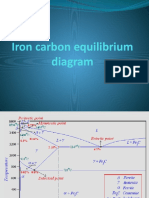
F e + liq uid D t1 H J B L iq uid S olid S o lutio n crysta ls t3 (A u sten ite) t4 E A u sten ite -iron Fe +A uste nite ( + ) A3 11 30 S o lidu s A u sten ite + L ed eb urite A1 K A u sten ite + C e m e n tite L iq uid u s L iq uid + C e m e n tite F E u te ctic C e m e n tite + L ed eb urite
t2 1 40 0 -Iro n + A u sten ite 1 30 0 1 20 0 11 00 1 00 0 G 9 00 8 00 7 23 7 00 -Iro n Fe 6 00 5 00 4 00 3 00 2 00 1 00 0 Q 0 .8 1 2 H ypo H ype re ute ctoid e ute ctoid S tee l Fe + P e arlite P e arlite + C e m e n tite S o lidu s
Tem peratureC
A cm
S P 0 .02 5% C a rbo n E u te ctoid
C e m e n tite + P e arlite + L ed eb urite
C e m e n tite + L ed eb urite
4 4 .3
6 .7
C a st Iro n Carbon Percentage
Fig. 8.6
Fe-C equilibrium diagram
8.6.1.1 Austenite Austenite is a solid solution of free carbon (ferrite) and iron in gamma iron. On heating the steel, after upper critical temperature, the formation of structure completes into austenite which is hard, ductile and non-magnetic. It is able to dissolve large amount of carbon. It is in between the critical or transfer ranges during heating and cooling of steel. It is formed when steel contains carbon up to 1.8% at 1130C. On cooling below 723C, it starts transforming into pearlite and ferrite. Austenitic steels cannot be hardened by usual heat treatment methods and are non-magnetic.
You might also like
- Ferrous Metals GuideDocument113 pagesFerrous Metals GuideAbhishek ChavanNo ratings yet
- Piping Engineering Guide: Materials, Components, Design & Stress AnalysisDocument103 pagesPiping Engineering Guide: Materials, Components, Design & Stress AnalysisJennifer FrenchNo ratings yet
- Unit 1 Heat Treatment of SteelsDocument207 pagesUnit 1 Heat Treatment of SteelsAishwarya JanbandhuNo ratings yet
- Timetal 834 Timet Data SheetDocument2 pagesTimetal 834 Timet Data SheetBonnie AttardNo ratings yet
- What Is Stainless Steel EN PDFDocument0 pagesWhat Is Stainless Steel EN PDFsssf-dobojNo ratings yet
- The Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalDocument38 pagesThe Iron-Carbon Phase Diagram: Prof. H. K. Khaira Professor in MSME Deptt. MANIT, BhopalYogesh KumbharNo ratings yet
- CIE 5100 Corrosion ExperimentsDocument25 pagesCIE 5100 Corrosion ExperimentsRaduku RaduNo ratings yet
- EN8 Steel: BS970: 1955 EN8, BS970/PD970: 1970 OnwardsDocument2 pagesEN8 Steel: BS970: 1955 EN8, BS970/PD970: 1970 OnwardsJohn MaldonadoNo ratings yet
- Piping Material SteelDocument44 pagesPiping Material SteelPPMNo ratings yet
- Documents - Pub - Lab Report Conductometric TitrationDocument14 pagesDocuments - Pub - Lab Report Conductometric Titrationsaa saNo ratings yet
- Ce Este Inox-UlDocument6 pagesCe Este Inox-UlIulian OlaruNo ratings yet
- Chapter 2Document42 pagesChapter 2Gila SutarNo ratings yet
- Fe-C Phase Diagram Explained for Low, Medium, High Carbon SteelsDocument7 pagesFe-C Phase Diagram Explained for Low, Medium, High Carbon SteelsSudhamsh KNo ratings yet
- Welding SS AusteniticDocument7 pagesWelding SS AusteniticAshish SinghNo ratings yet
- Chapter 5 - Welding of High-Alloy Steels, Corrosion PDFDocument19 pagesChapter 5 - Welding of High-Alloy Steels, Corrosion PDFEmad A.AhmadNo ratings yet
- 9 Engineering AlloysDocument17 pages9 Engineering AlloysdavidtomyNo ratings yet
- Everything You Need to Know About TitaniumDocument52 pagesEverything You Need to Know About TitaniumAi Heart PinkNo ratings yet
- Material PropertiesDocument93 pagesMaterial Propertiessachin_sawant1985No ratings yet
- Steel: CE 2330 Jul - Nov 2017 IIT TirupatiDocument32 pagesSteel: CE 2330 Jul - Nov 2017 IIT TirupatiUmar AlamNo ratings yet
- High Perf Metals Brochure v1Document5 pagesHigh Perf Metals Brochure v1arianaseriNo ratings yet
- IIC DiagramDocument57 pagesIIC DiagramAbhishek ChavanNo ratings yet
- Study of Microstructure of Steels at Different Cooling Rates and Further Check Hardness of The SamplesDocument43 pagesStudy of Microstructure of Steels at Different Cooling Rates and Further Check Hardness of The SamplesDeepu ChoudharyNo ratings yet
- Types of Thermocouples GuideDocument4 pagesTypes of Thermocouples GuidejordhyyNo ratings yet
- Electrochemical Series Table Reference GuideDocument10 pagesElectrochemical Series Table Reference GuideMycoLogist4LifeNo ratings yet
- 3 Fe-Fe3C Phase DiagramDocument33 pages3 Fe-Fe3C Phase DiagramRajat Mishra100% (1)
- Master In Metallurgy And Materials Science TALLER N°2Document7 pagesMaster In Metallurgy And Materials Science TALLER N°2ricardo alfonso paredes roaNo ratings yet
- Sulfuric Acid and Hydrochloric Acid Dew-Point Corrosion-Resistant SteelDocument0 pagesSulfuric Acid and Hydrochloric Acid Dew-Point Corrosion-Resistant SteelMatt AgonyaNo ratings yet
- Ceramo Metal AlloysDocument12 pagesCeramo Metal AlloysAna Maria de JesusNo ratings yet
- FALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramDocument33 pagesFALLSEM2019-20 MEE1005 ETH VL2019201001078 Reference Material I 29-Aug-2019 Fe-Fe3C Phase DiagramFazal KhanNo ratings yet
- Extraction of Titanium Dioxide from Ilmenite and Titaniferous Slag Using Fused Salt SolventsDocument6 pagesExtraction of Titanium Dioxide from Ilmenite and Titaniferous Slag Using Fused Salt SolventssecateNo ratings yet
- Metallurgy of Titanium and its Alloys ExplainedDocument8 pagesMetallurgy of Titanium and its Alloys ExplainedLowry GuettaNo ratings yet
- Metalurgia BásicaDocument30 pagesMetalurgia BásicaClever Ricardo ChinagliaNo ratings yet
- Salt Spray ChamberDocument13 pagesSalt Spray ChamberSijo Kaviyil JosephNo ratings yet
- Prepared By:-Sumant Sahu Metallurgy 3203808302Document21 pagesPrepared By:-Sumant Sahu Metallurgy 3203808302Rahul PandeyNo ratings yet
- Everything You Need to Know About TitaniumDocument20 pagesEverything You Need to Know About TitaniumAriska AndrainiNo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojNo ratings yet
- Gorni SFHTHandbookDocument128 pagesGorni SFHTHandbooktiberiusmanda8651No ratings yet
- C 10Document3 pagesC 10Michaelben MichaelbenNo ratings yet
- Titanium and Titanium AlloysDocument176 pagesTitanium and Titanium AlloysChaitrali DesaiNo ratings yet
- Diat HTT Lect-28Document12 pagesDiat HTT Lect-28prakush01975225403No ratings yet
- S-EMM 3122-CH5-Phase Diagram-Part DDocument15 pagesS-EMM 3122-CH5-Phase Diagram-Part DKHAIRUL NASHRAN BIN ANUAR / UPMNo ratings yet
- A 447Document27 pagesA 447superman3kNo ratings yet
- Mind Mapping TemplateDocument15 pagesMind Mapping TemplateSuriati Bt A RashidNo ratings yet
- Effect of Calcium and Magnesium Treatment On Steel WeldabilityDocument7 pagesEffect of Calcium and Magnesium Treatment On Steel WeldabilitySuleyman HaliciogluNo ratings yet
- ASSIGNMENT 3, OLeitch & JJarvis Mec 3206Document25 pagesASSIGNMENT 3, OLeitch & JJarvis Mec 3206Oneil Prettyboyswagg LeitchNo ratings yet
- Current Transformer GuideDocument79 pagesCurrent Transformer Guidesabill arasyid100% (1)
- Materials Science & Metallurgy: Titanium AlloysDocument13 pagesMaterials Science & Metallurgy: Titanium AlloysMarisa RobertsNo ratings yet
- Fe-Fe3C phase diagram analysisDocument10 pagesFe-Fe3C phase diagram analysisAlina OmerovicNo ratings yet
- Lesson 5 - Plain Carbon SteelsDocument4 pagesLesson 5 - Plain Carbon SteelsOwen GichangiNo ratings yet
- 3-12 - Heat TreatmentDocument10 pages3-12 - Heat TreatmentJasbir S RyaitNo ratings yet
- Application of Phase DiagramDocument29 pagesApplication of Phase DiagramAyu Sekar TunjungNo ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- Publication - Painting, Coating & Corrosion Protection - Atlas Steels - "L", "H" and STANDARD GRADES of STAINLESS STEELS Technical NoteDocument4 pagesPublication - Painting, Coating & Corrosion Protection - Atlas Steels - "L", "H" and STANDARD GRADES of STAINLESS STEELS Technical Notezinha_alNo ratings yet
- Iron-Carbide DiagramDocument6 pagesIron-Carbide DiagramAbhijit GhanwatNo ratings yet
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- High Temperature Corrosion: Fundamentals and EngineeringFrom EverandHigh Temperature Corrosion: Fundamentals and EngineeringNo ratings yet
- Price ListDocument3 pagesPrice ListchuranjitNo ratings yet
- How To Write Research PaperDocument3 pagesHow To Write Research PaperchuranjitNo ratings yet
- PDF in Authorized CentreDocument5 pagesPDF in Authorized CentrechuranjitNo ratings yet
- Bruce Treadmill Test: Time at ExhaustionDocument1 pageBruce Treadmill Test: Time at ExhaustionchuranjitNo ratings yet
- Past Tips and TechniquesDocument5 pagesPast Tips and TechniqueshamidurcanadaNo ratings yet
- Compression Molding GuidelinesDocument30 pagesCompression Molding GuidelinesadhityaNo ratings yet
- Kanomax Cabin Leakage TesterDocument1 pageKanomax Cabin Leakage TesterchuranjitNo ratings yet
- 5 PotentiometerDocument7 pages5 PotentiometerMadan YadavNo ratings yet
- Interior Product BrochureDocument32 pagesInterior Product BrochureyogaarsaNo ratings yet
- Lec25 CompositeDocument27 pagesLec25 CompositechuranjitNo ratings yet
- Chapter 1 IntroductionDocument8 pagesChapter 1 IntroductionS.h. Fahad FiazNo ratings yet
- Drafting Paper SizesDocument1 pageDrafting Paper SizeschuranjitNo ratings yet
- Tensile Testing of Polymer Matrix Composite Materials ASTM D3039, D3039MDocument2 pagesTensile Testing of Polymer Matrix Composite Materials ASTM D3039, D3039MchuranjitNo ratings yet
- Insert PDF in Catia V5Document3 pagesInsert PDF in Catia V5churanjitNo ratings yet
- Non TradDocument70 pagesNon TradHelliel OliveiraNo ratings yet
- 17.manufacturing TechnologyDocument16 pages17.manufacturing TechnologychuranjitNo ratings yet
- Mold-in Insert GuideDocument1 pageMold-in Insert GuidechuranjitNo ratings yet
- Appliance Design - July 2011Document36 pagesAppliance Design - July 2011churanjitNo ratings yet
- MadnessDocument16 pagesMadnesslovelytalkingsNo ratings yet
- JUMP36VDocument1 pageJUMP36VchuranjitNo ratings yet
- Snap Fit DesignDocument9 pagesSnap Fit DesignchuranjitNo ratings yet
- Excel 2003 Keyboard Shortcut KeysDocument3 pagesExcel 2003 Keyboard Shortcut KeysZorin GuraNo ratings yet