Professional Documents
Culture Documents
Modeling Osmosis
Uploaded by
api-208327322Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Modeling Osmosis
Uploaded by
api-208327322Copyright:
Available Formats
MODELING OSMOSIS (LAB) PROBLEM: Which way will water flow when a raw egg is placed in 250ml of vinegar?
HYPOTHESIS: If a raw egg is placed in vinegar (water) and left for four days, then the water will flow from the vinegar (high concentration) through the membrane into the egg (low concentration). THEORY: Osmosis is the diffusion of water from an area of high concentration to an area of low concentration across a selectively permeable membrane. 95% of the vinegar is water, and only 74% of the egg is water. Because the vinegar has a higher concentration of water, it will flow into the egg (which has lower concentration.)The acetic acid in the vinegar will dissolve the eggshell, leaving a selectively permeable membrane. This will allow the water from the vinegar to flow into the egg therefore causing osmosis. PROCEDURE: 1. Measure circumference of a raw egg using string and a ruler in cm. 2. Place egg in beaker and fill with 250 mL of vinegar. 3. Record observations. 4. Measure circumference for 4 days and record observations. DATA/OBSERVATIONS: DAYS 1 CIRCUMFERENCE (cm) 13.5 LIQUID LEVEL (Ml) Without 250 With egg 300 OBSERVATIONS 50ml displacement Bubbles collecting on cell. Some bubbles going to surface. The water displacement was 55ml. Feels slimy/rubbery. Shell almost fully dissolved. Foam around egg Yellow Smooth Huge
14.5
Without 225 280 with egg
16
With egg 275 Without 200
TOTAL EXPANSION GROUP #1= 2.5cm AVERAGE EXPANSION CLASS = 2.9cm AVERAGE EXPANSION (7th GRADE)= 2.7cm 100% of the eggs tested, expanded.
Koen, Timothy
Friday, October 11, 2013 10:14:27 AM Pacic Daylight Time
70:56:81:af:cd:55
AVERAGE EXPANSION COMPARISON
AVERAGE EXPANSION IN cm 3 2.9 2.8 2.7 2.6 2.5 2.4 2.3 Group 1 Period 3 7th Grade 2.5 2.9 2.7 Expansion Comparison
Koen, Timothy
Friday, October 11, 2013 10:14:27 AM Pacic Daylight Time
70:56:81:af:cd:55
CONCLUSION In this lab, we investigated the effects of osmosis on a selectively permeable membrane. The high concentration of water flowed from the vinegar into the egg, which had a low concentration of water, causing the egg to expand 2.5cm. This was only 0.2cm away from the 7th grade average expansion, and 0.4cm away from the class average. My hypothesis stated that the water molecules from the vinegar would go through the egg. My hypothesis was correct 100% of the time in which we tested the effects of osmosis on the egg.
ANALYSIS 1.The eggshell dissolved when the vinegar came in contact with the egg shell (calcium carbonate) this caused a chemical reaction. When this chemical reaction occurred, the eggshell started to dissolve, exposing the selectively permeable membrane of the egg. 2. The difference in expansion between the two groups is 3.4cm. The rate of expansion for group 3s egg was 1.125cm per day, group 7s was 0.275cm per day. These groups couldve gotten different results mainly because of the concentration of water in the egg. The concentration couldve been higher than 74% and the vinegars concentration lower than 94%. 3. Cells need water so that they can dilute chemicals, regulate their temperature, transport things throughout the cell, keep their shape, and water also takes place in some important chemical reactions in a cell.
Koen, Timothy
Friday, October 11, 2013 10:14:27 AM Pacic Daylight Time
70:56:81:af:cd:55
You might also like
- Cranes ExeDocument1 pageCranes Exeapi-208327322No ratings yet
- GeometricnestorismDocument7 pagesGeometricnestorismapi-208327322No ratings yet
- Legislativebilldraft 1Document3 pagesLegislativebilldraft 1api-208327322No ratings yet
- Dear Reporters Without BordersDocument1 pageDear Reporters Without Bordersapi-208327322No ratings yet
- Planaria Lab ReportDocument3 pagesPlanaria Lab Reportapi-208327322No ratings yet
- Koll Islandso Final 2Document30 pagesKoll Islandso Final 2api-208327322No ratings yet
- Uv Bead Lab 1Document3 pagesUv Bead Lab 1api-208327322No ratings yet
- Atomic Model ResearchDocument3 pagesAtomic Model Researchapi-208327322No ratings yet
- PurposeofgovtpresentationDocument10 pagesPurposeofgovtpresentationapi-208327322No ratings yet
- GeometricnestorismDocument2 pagesGeometricnestorismapi-208327322No ratings yet
- Circuit Write UpDocument3 pagesCircuit Write Upapi-208327322No ratings yet
- Chap3summary CopypdfDocument12 pagesChap3summary Copypdfapi-208327322No ratings yet
- PythagoreamtheoremDocument2 pagesPythagoreamtheoremapi-208327322No ratings yet
- A Perfect UtopiaDocument2 pagesA Perfect Utopiaapi-208327322No ratings yet
- Business LetterDocument1 pageBusiness Letterapi-208327322No ratings yet
- Summative ProjectDocument3 pagesSummative Projectapi-208327322No ratings yet
- PlatomonologueDocument3 pagesPlatomonologueapi-208327322No ratings yet
- Fictional NarrativeDocument6 pagesFictional Narrativeapi-208327322No ratings yet
- Will My Building Withstand Eq 2013Document3 pagesWill My Building Withstand Eq 2013api-208327322No ratings yet
- PlatomonologueDocument3 pagesPlatomonologueapi-208327322No ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Portfolio - Lesson - Thinking HatsDocument2 pagesPortfolio - Lesson - Thinking Hatsapi-231993252No ratings yet
- Rate CardDocument1 pageRate CardSalvato HendraNo ratings yet
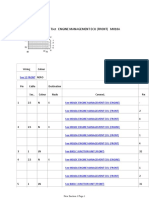
- Cylinder Head Valve Guide and Seat Inspection and RepairDocument1 pageCylinder Head Valve Guide and Seat Inspection and Repairnetifig352No ratings yet
- Preliminary Hazard Identification: Session 3Document19 pagesPreliminary Hazard Identification: Session 3Isabela AlvesNo ratings yet
- Understanding Future Tenses with "Be Going To" and "WillDocument11 pagesUnderstanding Future Tenses with "Be Going To" and "WillYasmín CastilloNo ratings yet
- Army C-sUAS Systems AssessmentDocument4 pagesArmy C-sUAS Systems AssessmentArthur WongNo ratings yet
- Canadair Regional Jet 100/200 - Automatic Flight Control SystemDocument28 pagesCanadair Regional Jet 100/200 - Automatic Flight Control Systemmamon113No ratings yet
- Oops NotesDocument79 pagesOops NotesaminNo ratings yet
- C# Abstract Classes and Interfaces ExplainedDocument20 pagesC# Abstract Classes and Interfaces ExplainedshubhamNo ratings yet
- Problems - SPCDocument11 pagesProblems - SPCAshish viswanath prakashNo ratings yet
- Biodegradabilty Prediction Using Deep LearningDocument9 pagesBiodegradabilty Prediction Using Deep LearningMadhuri DNo ratings yet
- ReportDocument329 pagesReportHovik ManvelyanNo ratings yet
- 2Ps2B-.85 High Pressure Oxygen Comressor: FeaturesDocument2 pages2Ps2B-.85 High Pressure Oxygen Comressor: FeaturesCastile100% (1)
- Chapters 1 and 3: ARM Processor ArchitectureDocument44 pagesChapters 1 and 3: ARM Processor ArchitectureTwinkle RatnaNo ratings yet
- Maintenance Manual: Models 8300, 8400, and 8500 Pallet Trucks and Model 8600 Tow TractorDocument291 pagesMaintenance Manual: Models 8300, 8400, and 8500 Pallet Trucks and Model 8600 Tow TractorJosé Luis Ang Soto92% (13)
- Construction Site Safety ReportDocument35 pagesConstruction Site Safety ReportAkshay Waim100% (1)
- Dune Supreme/ Dune Vector: WWW - Armstrong-Ceilings - Co.Uk WWW - Armstrong-Ceilings - IeDocument2 pagesDune Supreme/ Dune Vector: WWW - Armstrong-Ceilings - Co.Uk WWW - Armstrong-Ceilings - IeMuneer KonajeNo ratings yet
- Seah 1998Document299 pagesSeah 1998Wallison MedeirosNo ratings yet
- 2017-12-25 17:27:54Document3 pages2017-12-25 17:27:54Raghman JrNo ratings yet
- Specification (General) Road Concreting ProjectDocument66 pagesSpecification (General) Road Concreting ProjectMARK RANEL RAMOSNo ratings yet
- 02 - MDS System and ControlDocument55 pages02 - MDS System and Controlchinith100% (1)
- Introduction To Data Analytics - AnnouncementsDocument16 pagesIntroduction To Data Analytics - AnnouncementsAmit GuptaNo ratings yet
- Bhatia CPD 20001Document1 pageBhatia CPD 20001bilalaimsNo ratings yet
- Daikin Ceiling Suspended Air ConditioningDocument11 pagesDaikin Ceiling Suspended Air ConditioningWeb Design Samui100% (4)
- Mineral oil lifetime estimation using activation energyDocument5 pagesMineral oil lifetime estimation using activation energyvzimak2355No ratings yet
- PCBA MachineDocument62 pagesPCBA MachineSahara MalabananNo ratings yet
- Elearn - Ecu Pins PDFDocument6 pagesElearn - Ecu Pins PDFJavierPariNo ratings yet
- Investigation On Thermal Characteristic of Banana Waste Wood Sawdust Briquette - PUBLISHEDDocument7 pagesInvestigation On Thermal Characteristic of Banana Waste Wood Sawdust Briquette - PUBLISHEDku1zarinaNo ratings yet
- 2715 Top BracingDocument41 pages2715 Top BracingValentin-Madalin Voica100% (2)
- Uptime Detailed SimpleModelDetermingTrueTCODocument9 pagesUptime Detailed SimpleModelDetermingTrueTCOvishwasg123No ratings yet