Professional Documents
Culture Documents
Applied Termo 122424
Uploaded by
rjrahul25Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Applied Termo 122424
Uploaded by
rjrahul25Copyright:
Available Formats
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.3 Ericsson Cycle:
The Ericsson cycle consists of two isothermal and two constant pressure processes. The processes are:
Process 1-2: Reversible isothermal compression. Process 2-3: Constant pressure heat addition. Process 3-4: Reversible isothermal expansion. Process 4-1: Constant pressure heat rejection.
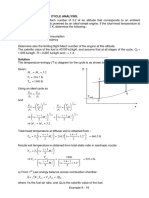
The heat addition and rejection take place at constant pressure as well as isothermal processes. Since the process 2-3 and 3-4 are parallel to each other on the T-s diagram, the net effect is that the heat need to be added only at constant temperature T3=T4 and rejected at the constant temperature T1=T2. The cycle is shown on p-v and T-s diagrams in Fig.4.3. The advantage of the Ericsson cycle over the Carnot and Stirling cycles is its smaller pressure ratio for a given ratio of maximum to minimum specific volume with higher mean effective pressure.
Volume
Indian Institute of Technology Madras
Gas Power Cycles
Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Temperature T3=T4
3 4
T2=T1
Entropy
Fig.4.3. Ericsson cycle on p-v and T-s diagrams
The thermal efficiency of Ericsson cycle is given by, (derivation is same as that of Stirling cycle),
th = TH - TL T = 1 - L TH TH
The Ericsson cycle does not find practical application in piston engines but is approached by a gas turbine employing a large number of stages with heat exchangers, insulators and reheaters.
Indian Institute of Technology Madras
You might also like
- Module 2 Combustion ThermodynamicsDocument29 pagesModule 2 Combustion ThermodynamicsDESHRAJ MEENA100% (2)
- Brayton CycleDocument6 pagesBrayton CycleNavnina Bhatia100% (1)
- Air Standard Cycles - BasicsDocument34 pagesAir Standard Cycles - Basicsrazvan66m100% (1)
- Stirling and Ericsson Cycles 2016 PDFDocument13 pagesStirling and Ericsson Cycles 2016 PDFArvin Loui Bascon100% (1)
- Theoretical CyclesDocument49 pagesTheoretical CyclesMariaEzzaSyUyNo ratings yet
- Quantum confinement effect bandgap relationshipDocument3 pagesQuantum confinement effect bandgap relationshipnirmalya prasun nayakNo ratings yet
- Important Trick To Remember All Thermodynamic Cycles For Oral ExamsDocument11 pagesImportant Trick To Remember All Thermodynamic Cycles For Oral ExamsParikshit Ugale50% (2)
- PN427448 Ansul Foam Manual PDFDocument342 pagesPN427448 Ansul Foam Manual PDFCHANDANNo ratings yet
- Activated Carbon, Silica-Gel and Calcium Chloride Composite Adsorbents For Energy Efficient Adsorption Cooling SystemsDocument136 pagesActivated Carbon, Silica-Gel and Calcium Chloride Composite Adsorbents For Energy Efficient Adsorption Cooling SystemsAlexander Vova100% (1)
- 3 CombustionDocument30 pages3 CombustionCllyan ReyesNo ratings yet
- Thermodynamics 2Document29 pagesThermodynamics 2S Tunkla EcharojNo ratings yet
- 2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxDocument7 pages2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxcaptainhassNo ratings yet
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringFrom EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringNo ratings yet
- The Carnot CycleDocument9 pagesThe Carnot CyclePatrick Antonio Orge ChingNo ratings yet
- 99041-Prediction of Scale and CO2 Corrosion in Oil Field SystemsDocument16 pages99041-Prediction of Scale and CO2 Corrosion in Oil Field SystemsdhireshmahajanNo ratings yet
- Design of Radial Turbines and TurbochargersDocument57 pagesDesign of Radial Turbines and Turbochargerstgsmech100% (1)
- Dispersed SystemsDocument112 pagesDispersed SystemsDawn WRein LegaspiNo ratings yet
- Diesel Cycle Efficiency and ProcessesDocument39 pagesDiesel Cycle Efficiency and ProcessesAchmad Rizal FirmansyahNo ratings yet
- Air Standard CycleDocument54 pagesAir Standard CycleKhurram SherazNo ratings yet
- Assignment and Its Solution - Airstandardcycle and VapourcycleDocument24 pagesAssignment and Its Solution - Airstandardcycle and VapourcycleMatthias100% (1)
- Edited Module1 ThermoDocument18 pagesEdited Module1 ThermoAnnaliza Alcazar ApostolNo ratings yet
- Characteristics of Laminar and Turbulent Pipe FlowDocument1 pageCharacteristics of Laminar and Turbulent Pipe FlowMohd Hafiz Ahmad100% (3)
- 2-The Variable LoadDocument14 pages2-The Variable LoadPotatoFryNo ratings yet
- Module 2. Properties and Characteristics of MaterialsDocument28 pagesModule 2. Properties and Characteristics of MaterialsPearl Alexandra FabitoNo ratings yet
- Shear and Moment DiagramsDocument25 pagesShear and Moment DiagramsJerico Pilapil Namuco100% (1)
- Parallel Line Method PDFDocument80 pagesParallel Line Method PDFalbertNo ratings yet
- Finals Learning Low-Speed Subsonic Wind Tunnel: Fundamentals OF AerodynamicsDocument9 pagesFinals Learning Low-Speed Subsonic Wind Tunnel: Fundamentals OF AerodynamicsJames Russell S. SanchoNo ratings yet
- Laboratory Activity 7 - Acceleration AnalysisDocument2 pagesLaboratory Activity 7 - Acceleration AnalysisDavid SaldivarNo ratings yet
- Uni ExamplesDocument23 pagesUni ExamplesAbhinash KumarNo ratings yet
- Aero Review ThermodynamicsDocument122 pagesAero Review ThermodynamicsKen GuanzonNo ratings yet
- Subject: Electrical Machines Conventional Practice QuestionsDocument9 pagesSubject: Electrical Machines Conventional Practice QuestionsPriya SharmaNo ratings yet
- Pumps 2016 PDFDocument15 pagesPumps 2016 PDF11212141No ratings yet
- Vibration TwoDocument20 pagesVibration TwoFeeling_so_flyNo ratings yet
- Machine Elements Quiz 1Document17 pagesMachine Elements Quiz 1Quen CuestaNo ratings yet
- Substation Solved ProblemsDocument50 pagesSubstation Solved ProblemsEli m. paredesNo ratings yet
- Strength of Materials Aug 5 2017Document16 pagesStrength of Materials Aug 5 2017MikaellaTeniolaNo ratings yet
- Bomb Calorimeter & Junkers CalorimeterDocument11 pagesBomb Calorimeter & Junkers Calorimeterlivillyle75% (4)
- Introduction to Kinematics of MachinesDocument3 pagesIntroduction to Kinematics of MachinesEmmanuelRomaresNo ratings yet
- MD ShaftDocument18 pagesMD Shaftiftikhar ahmedNo ratings yet
- Vapour Power Cycles Reheat Rankine CycleDocument12 pagesVapour Power Cycles Reheat Rankine CycleWilliam J ThompsonNo ratings yet
- Engineering Lab 5 Machine Lab Lab 1 ReportDocument9 pagesEngineering Lab 5 Machine Lab Lab 1 Reportram010No ratings yet
- Gas Turbine Problems and Efficiency CalculationsDocument2 pagesGas Turbine Problems and Efficiency CalculationsShobit Garg100% (1)
- DVD Mini Component System Troubleshooting and Repair GuideDocument69 pagesDVD Mini Component System Troubleshooting and Repair GuideAlbertoNo ratings yet
- Mechanical Engineering Laboratory 1 PlanimeterDocument8 pagesMechanical Engineering Laboratory 1 PlanimeterChristopher Lennon Dela CruzNo ratings yet
- Theories of failure: Maximum principal stress, shear stress and strain energyDocument2 pagesTheories of failure: Maximum principal stress, shear stress and strain energyBas KaranNo ratings yet
- Magnets and Electromagnetism FundamentalsDocument44 pagesMagnets and Electromagnetism FundamentalsRm NeneNo ratings yet
- Work and Heat in ThermodynamicsDocument129 pagesWork and Heat in Thermodynamicssohan2902No ratings yet
- Module-3 Laplace and Inverse Laplace Transforms PDFDocument45 pagesModule-3 Laplace and Inverse Laplace Transforms PDFPreetham N KumarNo ratings yet
- Mechanics of Deformable Bodies: Mapúa Institute of TechnologyDocument16 pagesMechanics of Deformable Bodies: Mapúa Institute of TechnologyAhsan AliNo ratings yet
- Turbine Types & Working ComparisonDocument13 pagesTurbine Types & Working Comparisonumarmanan100% (1)
- 1st Lec DC Circuits & Network TheormDocument88 pages1st Lec DC Circuits & Network Theormrktiwary256034No ratings yet
- 6 DC Machimes - DC GeneratorsDocument30 pages6 DC Machimes - DC GeneratorsJohn Rennier MandanasNo ratings yet
- Single Stage CompressorDocument5 pagesSingle Stage CompressorHaziq HanifahNo ratings yet
- Assignment 2Document5 pagesAssignment 2Noraishah Syahirah Azhar100% (1)
- ACE Engineering Academy Mock Test 1 Mechanical Engineering Paper 1Document15 pagesACE Engineering Academy Mock Test 1 Mechanical Engineering Paper 1vidya chakitwarNo ratings yet
- MEK451-Chapter - 2 - First - Law - of - Thermodynamics - M2Document68 pagesMEK451-Chapter - 2 - First - Law - of - Thermodynamics - M2MUHAMMAD DANISH HAZIQ AMIRULL FAKARUDDINNo ratings yet
- Force Vibration With Harmonic Excitation: Unit - IvDocument24 pagesForce Vibration With Harmonic Excitation: Unit - IvLathish KumarNo ratings yet
- Mechanics of Materials Course OutlineDocument252 pagesMechanics of Materials Course Outlinekhan janNo ratings yet
- Gas Power Cycles IITDocument68 pagesGas Power Cycles IITAfsar HusainNo ratings yet
- Carnot CycleDocument6 pagesCarnot CycleyuvrajsinghvloggingNo ratings yet
- 2 Carnot CycleDocument6 pages2 Carnot CyclecaptainhassNo ratings yet
- ThermoDocument105 pagesThermoAcfMacNo ratings yet
- Understanding the Stirling Cycle Gas Power ProcessDocument3 pagesUnderstanding the Stirling Cycle Gas Power ProcessRon QuerubinNo ratings yet
- Stirling and Ericsson Cycles 2016 PDFDocument13 pagesStirling and Ericsson Cycles 2016 PDFMarion Villamor100% (7)
- SESM2021: Introduction To Energy Technologies Coursework I: Student Name: Eleni Tsigkogianni 24467669Document8 pagesSESM2021: Introduction To Energy Technologies Coursework I: Student Name: Eleni Tsigkogianni 24467669inele1No ratings yet
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Document5 pagesQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VDocument7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VcaptainhassNo ratings yet
- Taking Birth Control Pills To Fight AcneDocument2 pagesTaking Birth Control Pills To Fight AcnecaptainhassNo ratings yet
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pagesQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhausDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhauscaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FDocument5 pages2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FcaptainhassNo ratings yet
- d dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)Document6 pagesd dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhausDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhauscaptainhassNo ratings yet
- Lecture 14Document8 pagesLecture 14captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NDocument8 pages2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellDocument6 pages2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellcaptainhassNo ratings yet
- Lecture 18Document6 pagesLecture 18captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXDocument8 pages2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document9 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CeDocument7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CecaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- N F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004Document6 pagesN F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document6 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) ADocument9 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) AcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: E HN MDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004: E HN McaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivityDocument5 pages2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivitycaptainhassNo ratings yet
- L-44 (GDR) (Et) ( (Ee) Nptel)Document15 pagesL-44 (GDR) (Et) ( (Ee) Nptel)yashaswiyellapragadaNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document8 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Problem Solving On D C Machines PDFDocument16 pagesProblem Solving On D C Machines PDFSelvaraj ParamasivanNo ratings yet
- Three-Phase Induction Motor: Version 2 EE IIT, KharagpurDocument8 pagesThree-Phase Induction Motor: Version 2 EE IIT, KharagpurHarsh PatelNo ratings yet
- 5 - AC Motor Starter PDFDocument10 pages5 - AC Motor Starter PDFPrem KumarNo ratings yet
- Equ. Circuit & Power Flow DiagramDocument7 pagesEqu. Circuit & Power Flow DiagramShareef KhanNo ratings yet
- SEO-Optimized Title for Physics Problems on Cylinder Suspended by Spring, Magnetic Field Induced EMF, and MoreDocument11 pagesSEO-Optimized Title for Physics Problems on Cylinder Suspended by Spring, Magnetic Field Induced EMF, and Moresanchit199617100% (1)
- Brayton Cycle With RegenerationDocument6 pagesBrayton Cycle With RegenerationMeshal Al-mutairi100% (1)
- Refractory Degradation in Glass Tank Melters. A Survey of Testing MethodsDocument5 pagesRefractory Degradation in Glass Tank Melters. A Survey of Testing MethodsRizqi Ahmad FauzanNo ratings yet
- HYSYS TutorialDocument30 pagesHYSYS TutorialEhsan Ahz100% (2)
- Giving Out Energy As ElectricityDocument2 pagesGiving Out Energy As ElectricityShahid Ur RehmanNo ratings yet
- ChemSepTutorial SimpleDistillationDocument27 pagesChemSepTutorial SimpleDistillationEvangelista LindaNo ratings yet
- Colloids PPT - PPTX 1Document22 pagesColloids PPT - PPTX 1Zhee ChoiNo ratings yet
- Juice Titration. Background. Acid - Base TitrationDocument11 pagesJuice Titration. Background. Acid - Base Titrationمقدم خالدNo ratings yet
- Mechanical Engineering Heat Transfer ChapterDocument30 pagesMechanical Engineering Heat Transfer ChapterFira tubeNo ratings yet
- Introductory Chemistry For Today 8th Edition Seager Test Bank 1Document11 pagesIntroductory Chemistry For Today 8th Edition Seager Test Bank 1shantel100% (40)
- The Process of DecaffeinationDocument3 pagesThe Process of Decaffeinationjrc5569No ratings yet
- Western Mindanao Chemistry WorksheetDocument2 pagesWestern Mindanao Chemistry WorksheetArvhenn BarcelonaNo ratings yet
- Ch. 1 Particulate Nature of MatterDocument10 pagesCh. 1 Particulate Nature of MatterهندNo ratings yet
- GUCET Syllabus and Books SuggestedDocument2 pagesGUCET Syllabus and Books SuggestedpremsempireNo ratings yet
- GS02 Remote ControlDocument52 pagesGS02 Remote ControlapakahsNo ratings yet
- FSC112 TEST COMPILATIONDocument15 pagesFSC112 TEST COMPILATIONRaphaelNo ratings yet
- Guo Et Al. - 2017Document9 pagesGuo Et Al. - 2017κ.μ.α «— Brakat»No ratings yet
- Preparation of Dye-Sensitized Solar Cells from TiO2 and Tamarillo ExtractDocument15 pagesPreparation of Dye-Sensitized Solar Cells from TiO2 and Tamarillo ExtractAbhishekNo ratings yet
- P-i-n Diode Working and TypesDocument9 pagesP-i-n Diode Working and TypesAshwin JoshiNo ratings yet
- Driving Equilibria: Dean-Stark Trap: Science Education CollectionDocument2 pagesDriving Equilibria: Dean-Stark Trap: Science Education CollectionLJ RBNo ratings yet
- GAC Regeneration Via FentonDocument235 pagesGAC Regeneration Via FentonNelson EnviromatchNo ratings yet
- Painting & Powder Coating ProcessesDocument5 pagesPainting & Powder Coating ProcessesdramiltNo ratings yet
- Organic chemistry notes on aldehydes ketones and carboxylic acidsDocument4 pagesOrganic chemistry notes on aldehydes ketones and carboxylic acidspoornaNo ratings yet
- Thermo LabDocument2 pagesThermo Labmuhyideen6abdulganiyNo ratings yet
- Atomic Models Seminar by Shanti SharmaDocument24 pagesAtomic Models Seminar by Shanti Sharmaahsanbgayo100% (1)