Professional Documents
Culture Documents
Reducere NOx
Uploaded by
Ghimis Simona BiancaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reducere NOx
Uploaded by
Ghimis Simona BiancaCopyright:
Available Formats
Korean J. Chem. Eng.
, 14(6), 491-497 (1997)
CATALYTIC REDUCTION OF NO OVER PEROVSKITE-TYPE CATALYSTS
Seong-Soo Hong*, Gun-Dae Lee*, Jong-Won Park**, Dae-Won Park**, Kyung-Mok Cho*** and Kwang-Jung Oh****
Department of Chemical Engineering, *Surface & Coating~Engineefing, Pukyong National University, 100 Yongdang-dong, Nam-ku, Pusan 608-739, Korea **Department of Chemical Engineering, *** Metallurgical Engineering, ****Environmental Engineering, Pusan National University, Pusan 609-735, Korea (Received 18 September 1997 9 accepted 14 November 1997)
Abstract- In the present work, we have investigated the reduction of NO by propane over perovskite-type oxides prepared by malic acid method. The catalysts were modified to enhance the activity by substitution of metal into A or B site of perovskite oxides. In addition, the reaction conditions, such as temperature, 02 concentration, and space velocity have been varied to understand their effects on the catalytic performance. In the LaCoO3 type catalyst, the partial substitution of Ba and Sr into A site enhanced the catalytic activity in the reduction of NO. For the Lao.6Ba (Sr)0.4 C01_,FexO3 (x=0-1.0) catalyst, the partial substitution of Fe into B site enhanced the conversion of NO, but excess amount of Fe decreased the conversion of NO. The surface area and catalytic activity of perovskite catalysts prepared by malic acid method showed higher values than those of solid reaction method. The conversion of NO increased with increasing 02 concentration and contact time. The introduction of water into reactant feed decreased the catalytic activity but the deactivation was shown to be reversible over Lao.6Bao.4Cox_,Fe,O3catalyst.
Key words: Reduction of NO, Perovskite-type Oxides, Malic Acid Method, Effect of Water, Substitution of Ba, Sr and Fe, TPR
INTRODUCTION There is a worldwide effort to discover improved solutions for the removal of NOx emissions [Armor, 1995]. Nitrogen oxides, N O , are major air pollutants that cause photochemical smog formation and acid rain. The emission of various nitrogen oxides into our atmosphere occurs on a massive scale. Worldwide, over 30 million tons of NO, are vented to the earth's atmosphere each year. The public is demanding greater control of the emissions of toxic NOx into our atmosphere. It is well known that the major source of NOx emissions is fuel combustion in automobile engines. Currently, we demands for improved fuel economy and reduced exhaust emissions. Therefore, de-NOx catalysts, or new aftertrealment systems for the removal of NOx gases which function also in an oxidizing atmosphere, have to be developed. The reduction of NO~ with hydrocarbons present in the feedstream has drawn attention as a process for the catalytic reduction of NO in the exhaust gas of automobile engines [Iwamoto et al., 1986; Nam, 1995]. Among the catalysts which have been reported, Cu-ion exchanged ZSM-5 is potentially the most effective catalyst for the reduction of NO with hydrocarbons [Iwamoto et al., 1991]. Unfortunately this catalyst shows very poor thermal durability, losing much of the catalytic activity after aging under real exhaust gas conditions on diesel or leanbum gasoline engines [Monroe et al., 1993]. In addition, Co*To whom all correspondence should be addressed. E-mail: sshong@pine.pknu.ac.kr 491
ZSM-5 does offer comparable activity with higher hydrocarbons, and it does appear to be much more hydrothermally stable[Armor et al., 1995]. Alumina-based catalysts for the NOx removal in an excess of oxygen are examined [Iwamoto et al., 1991]. In the development of automotive catalysts, the thermal stability should be essential for a practical application to high-performance automobile engine systems. It was reported that the study of NO reduction with methane over La203 has been examined under an excess oxygen [Zhang et al., 1994]. The metal oxide catalyst shows high thermal stability but poor activity of NO, reduction. Perovskite oxides, which are structurally similar to the mineral of that name (CaTiO3), have long been studied because of technologically important physical characteristics. In 1971, cobaltate perovskites were suggested as substitutes for noble metals in automotive exhaust catalysis [Libby, 1971]. More recently, Teraoka et al. have studied the simultaneous removal of NO and diesel soot over perovskite oxides [Teraoka et al., 1996]. In the present work we study the properties of perovskitetype oxides prepared by malic acid and solid reaction method. We also examine the catalytic activity for NO reduction with propane in the presence of oxygen and the effect of reaction conditions, such as residence time, reaction temperature, especially the effect of water introduced into the feed stream. The studies on the catalytic reduction of NO will be extended additional modified perovskite-type oxides for the simultaneous removal of diesel soot.
492 EXPERIMENTAL
S.-S. Hong et al.
50
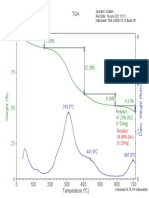
It is well known that the catalytic activity differs according to the preparation method. In this study, we prepared two type of perovskite oxides in order to compare the property of catalysts according to the preparation method. At first in the malic acid method, malic acid was added into mixed aqueous solution of metal nitrates in a desired proportion so as for the molar ratio of malic acid to the total metal cations to be unity. The mixed aqueous solution was controlled at pH 4.0 by addition of ammonia water and then evaporated to dryness with stirring, followed by drying at 150 ~ The precursor was followed by grinding, calcination in air at 200 ~ for haft h and 350 ~ for half h and 600 ~ for 12 h. The calcination temperature was determined by the result of thermal analysis of precursors. In addition, the solid reaction method was followed by the literature [Teraoka et al., 1991]. Metal oxides were dried at 120 ~ for 2 h and were mixed with Co(NO3)~ and Fe(NO3)2. The mixture was grinded in an agate mortar and then was calcined at 1,050 ~ for 12 h. The crystal structures of prepared oxides were examined by powder X-my diffraction (XRD) with Cu K~x radiation (Rigaku Co. Model DMax) and the BET surface area was measured using BET measuring apparatus (Quanta Chrome Surface Area Analyzer). The sample was pretreated at 200 ~ for 1 h before N2 adsorption. The dead volume of sample port was measured with He gas. In addition, Temperature Programmed Reduction (TPR) was carded out using TCD type detector cell. Reaction tests were carried out in a continuous flow fixedbed reactor. The reaction gases, NO (5,000 ppm), C3H8 (2,000 ppm), O2 (14.6 %), He balance, were used. The reactor was made of quartz tube, I.D. of 1.0 cm and 24 cm long, mounted in a tubular furnace. The reaction was carded out at the range of 250-400 ~ the exhaust gas stream temperature. The water was introduced to the system by flowing helium through a gas dispersion tube in a glass saturator containing the liquid. The reactant composition, NO (1,000 ppm), C3H8 (1,000 ppm), 02 (4 %), was controlled by the Helium as a diluent gas. The outlet gas was analyzed by NOx analyzer. RESULTS AND DISCUSSION 1. Characterization of Catalysts After the drying process of the mixed solution of metal nitrates and malic acid at 150 ~ precursor is obtained by following the sol-gel state. If this precursor calcinated at 600 ~ directly, a lot of impurities, such as SrCO3, is able to be mixed in the catalyst. It is thought that impurities due to reaction of Sr ion with CO or COz which is formed by decomposition of malic acid all at once. Thus we have to calcinate the precursor stepwise to prevent the formation of impurities. Fig. 1 shows the thermogravimetric analysis of powder precursor in air. TGA peak shows the decrease of weight three times at the range of 150-280~ 330-400~ and 530620 ~ They are caused by decomposition of NO3 and free malic acid, malic complex and Sr(NO3)2 or Sr(CO3)2 respectively. This result is confirmed by literature for the citric acid-aided process [Anderson et al., 1979]. The last drop plays November, 1997
4O
\
5-1
30
-2
/
I
0 200
10
I
400 Teml~rature
I
600 800
-10
(4C)
Fig. 1.Thermogravimetric analysis and differential thermal analysis curves for the precursor of L a o . 6 S r o A C o o ~ F e o . 2 03: heating rate=10 ICmin.
LaCoO~
<
t., jea,.,COo. FeuOs
II
i I
80
20
4O
60
2-Theta (deg)
Fig. 2.XRD patterns of perovskite catalysts prepared by malic acid method. an important role in the crystallization of perovskite oxide followed by removal of excess oxygen [Jonker et al., 1953]. Therefore it is important to give enough reaction time in the formation of perovskite structure and the precursor was calcinated at 200 ~ for half h, at 350 ~ for half h and at 600 ~ for 12 h. The crystal structures of prepared oxides were examined by XRD and the results are shown in Fig. 2. In spite of preparation method, XRD patterns show large peak at 33 ~ and confirm the formation of perovskite crystalline phase [Anderson et al., 1979]. The surface area of perovskite oxides was measured by BET measuring apparatus. Table 1 shows the surface areas of catalysts by different preparation method. While small surface area is obtained about 1 m2/g in the catalyst prepared by solid reaction method, large surface areas more than 10 m2/g are obtained in the catalyst prepared by malic acid method. It is
Catalytic Reduction of NO over Perovskite-Type Catalysts
Table 1. BET surface areas of various perovskite catalysts
100
493
Catalyst LaCoOa
Preparation method Malic acid La0.6Sr0.4CoO 3 Malic acid Lao.6Sr0.4FeO3 Malic acid Lao.6Sr0.4fo0.8Fe0,203 Malic acid t~.6Ba0.4CoO3 Malic acid Lao.rBa0.4FeO3 Malic acid Lao.6Bao.,~Coo.sFr Malic acid Lao.6Bao.4Co0_sFe0503 Malic acid LaCoO3 Solid state rxn Lao.6Bao.4Coo_sFe~O3 Solid state rxn Lao6Sro.4CoO3 Solid state rxn Lao.6Sro4FeO3 Solid state rxn
Calcining Surface area conditions (mZ/g) 600 ~ 12 hr 4.0 600 ~ 12 hr 8.8 600 ~ 12 hr 57.8 600 ~ 12 hr 20.0 600~ 12 hr 5.7 600 ~ 12 hr 26.5 600 ~ 12 hr 7.9 600 ~ 12 hr 12.2 1,050~ 12 hr 1.1 1,050~ 12 hr 0.8 1,050 ~ 12 hr 0.8 1,050 ~ 12 hr 1.2
80 60
Q
> 0
40
2O 0 ~lll~-e-
..... e - ..... e - ..... e - ..... 9
10
20
30
40
50
60
thought that the latter is calcinated at lower temperature than the former because malic acid easily makes the complex with transition metal and then it stabilizes the perovskite oxide obtained by the complex at low calcination temperature. In addition, the addition of Fe into B site increases surface area.
2. Effect of Substitution of Metal Ion into A Site
Time(rain)
Fig. 3. The effect of reaction time on the conversion of NO over Lao.6Sre.4Coo.sFeoaO3; NO=l,000 ppm, C3Hs=I,000 ppm, O2=4%, GHSV=30,000 hr -1"
Fig. 3 shows NO conversion with time on stream over Lao.6Sr0.4Co0.sFe0.zO3. Steady state is obtained relatively fast at almost reaction temperature. In addition, steady state reaches within 20 minute at 300~ and the activity shows almost constant values. Thus the value of NO conversion is obtained after 1 hr reaction. Fig. 4 shows the effect of substitution of metal into A site of LaCoO3. The substitution of Sr or Ba into A site of LaCoO3 increases NO conversion. The general formula of perovskites is ABO3. Their crystal structure is relatively simple. The B ions may be catalytically active 3d, 4d or 5d transition metal ions which occupy octahedron. The A ions, which fit into dodecahedral interstices, may be large rare-earth, alkaline-earth, alkali or large ions. Even for a single active metal B center, there is still the freedom to vary its valence and many physical properties by choices of the modifying A ion. In Lal_xSrxCoO3, oxygen vacancies compensate for the rising Sr content beyond x=0.4 [Junker et al., 1953]. One phase perovskites LaNiO3-)~ (~._< 0.25) have been described but are not very stable. The reduction of NO by CO and H2 over perovskite-type catalysts has been classified as an intrafacial process [Voorhoeve et al., 1976]. It was well known that NO adsorbs more strongly on reduced transition metal oxides on oxidized sample [Yao et al., 1973]. An example of an intrafacial process is the reduction of NO, which occurs through dissociative chemisorption on an oxygen vacancy of the perovskite surface. NO+[M-Vo-M] ---* Nad~+[M-O-M] In this reaction, the reduced and oxidized surfaces are symbolized by [M-Vo-M] and [M-O-M], respectively. No,, the resuiting N fragment, which probably has to be stabilized by interaction with other adsorbates, may react further to form N20 and N2 and so forth.
-O-: 250~ - 0 - : 375 ~
- i - " 275 ~ -A-: 300 ~ ..O-: 400 ~
-V-: 325 ~
4-:
'~176 t E
40
0 250 r 275 i 300 i 325 i 350 i 375 r 400
8O
Tmi~ratare('C)
Fig. 4. T h e effect of reaction temperatm'e on the conversion of NO over perovskite catalysts; NO=l,000 ppm, C~Hs =1,000 ppm, 02=4 %, GHSV=30,000 hr -1.
Na~+NO~ ~ NzO 2Nad~ ---* N2 The Vo is restored by a reducing agent, hydrocarbon, in the gas, [M-O-M]+HC--- [M-Vo-M]+CO, CO2 It is understandable from the mechanism that the number of oxygen vacancy in the surface is important for the rate of NO conversion. It is thought that the number of Vo increases and then NO conversion also increases in the substitution of Sr or Ba into A site.
Korean J. Chem. Eng.(Vol. 14, No. 6)
494
S.-S. Hong et al.
100
80-
i,,,,;-.>. ,,
o 40
,/
i
/
(b)
~ _.~__
i
Lao-tSro-4C~
Lao.6Sro.4Coo.65Feo.3503
i t i '
200
400
600
800
250
275
300
325
350
375
400
Temperature (*(2) Fig. 5. TPR profiles "measured for various perovskite type oxides calcined at 600oC for 12 hrs (Heating rate=10 K/min, gas mixture=7.5 % H2/N2). (a) LaCoO3, (b) tao.6na0.4CoO3, (c) La0.6Sr0.4CoO3 Fig. 5 shows the TPR results of various perovskite oxides. The LaCoO3 shows two kinds of reduction peaks at 400 ~ and 610 ~ respectively but in the substitution of Sr or Ba, the peak size increases at low temperature and disappears at high temperature. This results suggests that the substitution of Sr or Ba gives rise to easy reduction of oxides and form oxygen vacancies of surface and then increase the catalytic activity of NO reduction. 3. Effect of Substitution of Metal Ion into B Site Fig. 6 shows the effect of substitution of Sr into A site and Fe into B site of LaCoO3. The substitution of Sr into A site enhances catalytic activity and that of Fe into B site also enhances the activity at the substitution ratio=0.2 but catalytic activity decreases at substitution ratio=0.35. In addition, the maximum activity appears at the range of 300-325 ~ over all catalysts. It was well known that oxygen ion moves through the lattice vacancy in the perovsldte-type oxides and the mobility of ion increases and then the activation energy of anion mobility decreases as the lattice vacancy increases [Cook et al., 1991]. Consequently the oxygen vacancies increase followed by removal of oxygen by reducing agent at surface as the mobility of oxygen ion increases in the lattice. In the TPR results of La0.6Sr0.4COl-xFexO3,the addition of Fe gives rise to move the peak position of ct-oxygen species to low temperature [Moon et al., 1996]. It is reported that the ix-oxygen species are not only strongly bonded to Co or Fe but reversible oxygen species which are concemed in reduction of oxygen by freely moving in the lattice of metal oxides. Therefore the expansion of lattice by addition of Fe gave rise to easily occur the reduction of oxygen by lowering the bond energy between tx-oxygen species and metal [Moon et al., 1996]. With the increase of Fe content, it is difficult to reduce oxygen owing to other factors except for lattice expansion. November, 1997
Temperature(*(;)
Fig. 6. The effect of reaction temperature on the conversion of NO over perovskite catalysts: NO=l,000 ppm, C3Hs =1,000 ppm, 02=4 %, GHSV=30,000 hr -1. Moon et al. reported that the content of irreversible oxygen increased with the increase of Fe content followed by holding the peripheral oxygen in the lattice [1996], and then ixoxygen species decreased relatively. As is shown in Fig. 6, the catalytic activity decreases according to the decrease of tzoxygen species in the Fe content-->0.35. Fig. 7 shows the effect of substitution of Ba into A site and Fe into B site of LaCoO3. The substitution of Ba into A site also enhances catalytic activity and that of Fe into B site shows the maximum activity at the substitution ratio=0.5. It can be explained as same reason before. In addition, the high activity appears at large range temperature compared to La0.6Sr0.4fol-xFexO3.This result suggests that Lao.6Bao.4COl-xFe~O3catalyst is better in the real
100
80
A4--~
6o
> g~
~" / ":
_ ~.~
".~
| o|
l I L
-v- ~,~=co=F%o, - + t~=~d~=~
l l | I
I [
400
250
275
300
325
350
375
Temperature (*C) Fig. 7. The effect of reaction temperature on the conversion of NO over perovskite catalysts; NO=l,000 ppm, C3Hs=l,000 ppm, 02=4 %, GHSV=30,000 hr -1.
Catalytic Reduction of NO over Perovskite-Type Catalysts
100 lO0
(
495
8O
v
e~ o
6O
0 0 Z
40
40-
2O
20-
la~Sr~CouFe u 3(S-S) ~C%sFeuO3(S-S)
0
250
, 275
, 300
, 325
I 350
l 375
400
Temperature~C)
Fig. 8. The effect of reaction temperature on the conversion of N O over perovskite catalysts; N O = l , 0 0 0 ppm, C3Hs =1,000 ppm, 02=4 %, GHSV--30,000 hr -t, M-A: malic acid mothod, S-S: solid reaction method.
02 Concentration (%) Fig. 9. The effect of oxygen concentration on the N O conversion over Lao.~Bao~Coo.sFeo~O3 catalyst: N O = l , 0 0 0 ppm,
C3l-ls=l,000 ppm, TR=350 ~
lOO
exhaust gas condition than La0.6Sr0.4COl_xFexO3. 4. Effect of Preparation Method of Catalysts It is well known that the catalytic activity differs according to the preparation method. In this study, we prepared two type of perovskite oxides in order to compare the property of catalysts according to the preparation method. The result is shown in Fig. 8. The catalyst prepared by malic acid method shows higher activity than that prepared by solid reaction method. In addition, the former shows the maximum activity at lower reaction temperature compared to the latter (350 ~ It is thought that the active site of the former increases owing to their high surface area. Therefore this result suggests that the catalyst prepared by malic acid method can be used effectively in the reduction of NO.
5. Effect of Reaction Conditions
o
t* o
C
4o
O z 20
-0.....
"
600O0
12000O
180000
240000
GHSV (hr"1)
It is well known that NO reduction is strongly promoted by oxygen [Iwamoto et al., 1991]. In Fig. 9, the effect of oxygen concentration on the NO conversion over Lao.6Bao.4COl-xFexO3 is shown. As is shown, the addition of oxygen into the gas feed has a dramatic effect on the NO conversion performance of the catalyst. This result suggests that the oxygen plays an important role on the reduction of NO. The similar results were shown by other authors [Montreuil et al., 1992; Monroe et al., 1993]. It is thought that partially oxygenated hydrocarbon species are capable of reducing NO under oxidizing conditions. In addition, such partially oxygenated species perform NO conversion in the absence of gas phase molecular oxygen. NO conversion increases with increasing of O2 concentration, but it is expected that NO conversion will decrease at high Oz concentration owing to decreasing of hydrocarbon. The effect of space velocity on the NO conversion over Lao.6Ba0.4COl_xFe~O3 is examined and the result is shown in Fig. 10. In this case, different space velocity was obtained by changing the mass of catalyst while holding the volumet-
Fig. 10. The effect of space velocity on the NO conversion over Lao.6Bao.4Co0.sFeo.sO3 catalyst: NO=I,000 ppm, C3I-I8= 1,000 ppm, TR--350 ~ Oz=4 %. ric flow rate constant. The NO conversion decreases with increasing space velocity, that is, deceasing contact time between catalyst and reactants. The similar results are shown over almost metal oxide catalysts [Torikai et al., 1991]. Therefore in real exhaust gas condition, the catalytic activity on the space velocity is considered to be very important. 6. Effect of Water Introduced into Feed Gas Since any combustion process is going to produce almost 16 % water vapor, one must focus on a Catalyst that is stable for long times in such wet environments. However no outstanding catalyst has been known to be stable in such conditions. We examined the effect of water on the catalytic activity and the result is shown in Fig. 11. The catalytic activity decreases under the wet environment over perovskite-type oxides. However La0.6Bao4fol_xFexO3 catalyst shows to have highKorean J. Chem. Eng.(Voi. 14, No. 6)
496
S.-S. Hong et ft. than those of solid reaction method. 4. In the Lao.6Ba(Sr)o.4Col_xFe~O3catalyst, the conversion of NO increased with increasing Oz concentration and contact time. 5. The introduction of water into reactant feed decreased the catalytic activity but the deactivation was shown to be reversible over Lao,6Bao.aCol_xFexO3catalyst.
w ~ n2o
80
.[ 6o
o
40
ACKNOWLEDGMENT The authors were to thanks the Institute of Environmental Technology and Industry of Pusan National University and Samsung Heavy Industries Co., LTD for providing financial support.
600
9 20 9
LauSro.4Co0.~et203 Lae.6Bao.4CotsFetsO 3
100
200
300
400
500
REFERENCES Armor, J. N., "Catalytic Removal of Nitrogen Oxides: Where are the Opportunities. , Catal. Today, 26, 99 (1995). q' Anderson, D.J. and Sale, F.R.,"Production of Conducting Oxide Powders by Armophous Citrate Process", Powder MetalL, 2L, 14 (1979). Cook, R. L and Sammuells, A. F., Solid State Chem., 14, 395 (1975). Iwamoto, M., Furikawa, H., Mine, Y., Uemura, F., Mikuriya, S. and Kagawa, S., J. Chem. Soc., Chem. Commun., 1272 (1986). Iwamoto, M. and Hamada, H., Catal. Today, 10, 57 (1991). Iwamoto, M. and Yahiro, H., Catal. Today, 22, 5 (1994). Jonker, G. H. and Van Santen, J. H., Physica, 19, 120 (1953). Li, Y. and Armor, J. N., "Selective Catalytic Reduction of NOx with Methane over Metal Exchanged Zeolites", AppL CataL, B, 2, 239 (1993). Libby, W. F., "Promising Catalyst for AutoExhaust', Science, 171, 499 (1971). Monroe, D. R., Dimaggio, C. L., Beck, D. D. and Matekunas, F. A., SAE 930737 (1993). Montreuil, C. N / a n d Shelef, M., Appl. Catal., B, 1, L1 (1992). Moon, H. D. and Lee, H.-I., "A Study on the Catalytic Characteristics of Oxygen Reduction in the Alkaline Fuel Cell", J. Kor. Ind. & Eng. Chem., 7(3), 554 (1996). Nam, I.S., "A Catalytic Process for the Reduction of NOx from Stationary Sources", Catalysis, 11, 5 (1995). Obayashi, H. and Kudo, T., Jpn. J. AppL Phys., 14, 330 (1975). Sato, S., Yu, Y., Yahiro, H., Mizuno and Iwamoto, M., "CuZSM-5 Zeolite as Highly Active Catalyst for Removal of Nitrogen Monoxide from Emission of Diesel Engines", Appl. Catal., 70, L1 (1991). Shangguan, W. F., Teraoka, Y. and Kagawa, S., "Simultaneous Catalytic Removal of NOx and Diesel Soot Particulates over Ternary ABzO, Spinel-type Oxides", Appl. Catal., B, 8, 217 (1996). Teraoka, Y., Hakebayzshi, H., Moriguchi, I. and Kagawa, S., "Oxygen-Sorptive Properties and Direct Structure of Perovskite-Type Oxide", Chem. Lett., 673 (1991). Torikai, Y., Yahiro, H., Mizuno, N. and Iwamoto, M., Catal.
Time (rain) l~g. ll.Reversibility of perovsldte catalyst deteriorated by water vapor: NO=l,000 ppm, C31-18=1,000 ppm, 02=4 % 1-120 =5 vol%, TR--325~
er stability than I.,ao.6Sr0.aCol-xFexO3catalyst. It is thought that the former has higher resistance to water vapor than other reported catalyst. It is well known that added water prohibits reactants from reacting with active metal by dealumination of the skeletal structure of zeolites [Iwamoto et al., 1994]. Actually, this weakness has blocked the practical use of zeolite catalyst. It is known that added water has different function in the metal oxide compared to zeolite [Nam et al., 1995]. It was reported that water prohibited from adsorption of reactants followed by competitive adsorption with reactants in the active site. In the perovskite-type oxide, it is thought that water in the reactants also decreases the active site of catalyst. In addition, it was reported that this deactivation was reversible, that is, the activity was recovered in the stoppage of water introduction [Nam et al., 1995]. As is shown in Fig. 11, the initial activity of tao.6Ba0.4co l_x FexO3 catalyst is almost recovered compared to Lao.6Sro.4Co~_x FexO3 catalyst. This suggests that deactivation by water is reversible over Lao.6Bao.4col_xFexO3catalyst. In addition, it is thought that Lao.6Bao.4COl-~Fe~O3catalyst deserves to be commercialized in the removal of NO~. CONCLUSIONS The following results are drawn from the reduction of NO by propane over perovskite-type oxides. 1. In the LaCoO3 type catalyst, the partial substitution of Ba, Sr into A site enhanced the catalytic activity in the reduction of NO. 2. In the La0.6Ba(Sr)0.,Coi_xFexO3 (x=0-1.0) catalyst, the partial substitution of Fe into B site enhanced the conversion of NO, but excess amount of Fe decreased the conversion of NO. 3. The surface area and catalytic activity of perovskite catalysts prepared by malic acid method showed higher values
Novembe~ 1997
Catalytic Reduction of NO over Perovskite-Type Catalysts
497
Lett., 9, 91 (1991). Voorhoeve, R. J. H., Remeika, J. P. and Trimble, L. E., "Perovskites Containing Ruthenium as Catalysts for Nitric Oxide Reduction", Ann, N.Y. Acad. Sci., 272, 3 (1976). Yao, H. C. and Shelef, M., "Surface Interaction of Oxygen
and Nitric Oxide with Mangneous Oxide", J. Catal., 31, 377 (1973). Zhang, X., Waletrs, A. B. and Vannice, M. A., "NO Decomposition and Reduction by Methane over La203', Appl. Catal., B, 4, 237(1994).
Korean J. Chem. Eng.(Vol. 14, No. 6)
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- NVH Ford PDFDocument188 pagesNVH Ford PDFReshma Mohamed71% (7)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Forces and Equilibrium QuestionsDocument3 pagesForces and Equilibrium QuestionsIshwarya Sivakumar100% (3)
- 5 Analysis of of Gravity DamsDocument10 pages5 Analysis of of Gravity DamsBillie Ian. Salamante JrNo ratings yet
- Wet cleaning of historical textiles: surfactants and other wash bath additivesDocument32 pagesWet cleaning of historical textiles: surfactants and other wash bath additivesJC4413No ratings yet
- Theory of CompressorsDocument43 pagesTheory of Compressorsmujeebtalib100% (3)
- Gschneidner K. A., Eyring L. - Handbook On The Physics and Chemistry of Rare Earths Vol. 20 (1995) (470s) PDFDocument472 pagesGschneidner K. A., Eyring L. - Handbook On The Physics and Chemistry of Rare Earths Vol. 20 (1995) (470s) PDFxxalikayaxxNo ratings yet
- Material Data Sheet: Ultrachrome® High CR, Low CDocument3 pagesMaterial Data Sheet: Ultrachrome® High CR, Low CfendixNo ratings yet
- Portal Frame PDFDocument2 pagesPortal Frame PDFtwinztubeNo ratings yet
- Vallorec P91 BOOKDocument69 pagesVallorec P91 BOOKhassan100% (2)
- Applied Catalysis A, General: SciencedirectDocument7 pagesApplied Catalysis A, General: SciencedirectGhimis Simona BiancaNo ratings yet
- Biomasa Alge.001Document1 pageBiomasa Alge.001Ghimis Simona BiancaNo ratings yet
- HTL Pilot Plant ProposalDocument6 pagesHTL Pilot Plant ProposalGhimis Simona BiancaNo ratings yet
- Applied Catalysis B: Environmental: Juan Martínez, Alfredo Ortiz, Inmaculada OrtizDocument18 pagesApplied Catalysis B: Environmental: Juan Martínez, Alfredo Ortiz, Inmaculada OrtizGhimis Simona BiancaNo ratings yet
- PDFDocument295 pagesPDFjerinNo ratings yet
- 1 s2.0 S0016236120321864 MainDocument7 pages1 s2.0 S0016236120321864 MainGhimis Simona BiancaNo ratings yet
- CO Oxidation On Mesoporous SBA-15 Supported CuO-CeDocument10 pagesCO Oxidation On Mesoporous SBA-15 Supported CuO-CeGhimis Simona BiancaNo ratings yet
- Waste Management: Chao-Yi Hung, Wen-Tien Tsai, Jie-Wei Chen, Yu-Quan Lin, Yuan-Ming ChangDocument8 pagesWaste Management: Chao-Yi Hung, Wen-Tien Tsai, Jie-Wei Chen, Yu-Quan Lin, Yuan-Ming ChangGhimis Simona BiancaNo ratings yet
- Mesopore Modified Mordenites As CatalystDocument30 pagesMesopore Modified Mordenites As CatalystGhimis Simona BiancaNo ratings yet
- NOVA - BrosuraDocument8 pagesNOVA - BrosuraGhimis Simona BiancaNo ratings yet
- Review Article: SciencedirectDocument16 pagesReview Article: SciencedirectGhimis Simona BiancaNo ratings yet
- Applied Catalysis B: Environmental: Juan Martínez, Alfredo Ortiz, Inmaculada OrtizDocument18 pagesApplied Catalysis B: Environmental: Juan Martínez, Alfredo Ortiz, Inmaculada OrtizGhimis Simona BiancaNo ratings yet
- Bio-Oil Produced Via Catalytic Pyrolysis of The Solid Digestates From Anaerobic Co-Digestion PlantsDocument2 pagesBio-Oil Produced Via Catalytic Pyrolysis of The Solid Digestates From Anaerobic Co-Digestion PlantsGhimis Simona BiancaNo ratings yet
- Study of Algae Pyrolysis for Bio-Oil and Bio-Char ProductionDocument7 pagesStudy of Algae Pyrolysis for Bio-Oil and Bio-Char ProductionGhimis Simona BiancaNo ratings yet
- Biomasa Alge.001Document1 pageBiomasa Alge.001Ghimis Simona BiancaNo ratings yet
- Study of Algae Pyrolysis for Bio-Oil and Bio-Char ProductionDocument7 pagesStudy of Algae Pyrolysis for Bio-Oil and Bio-Char ProductionGhimis Simona BiancaNo ratings yet
- Catalytic Conversion of Bamboo Sawdust o PDFDocument10 pagesCatalytic Conversion of Bamboo Sawdust o PDFGhimis Simona BiancaNo ratings yet
- Biomasa Alge.001Document1 pageBiomasa Alge.001Ghimis Simona BiancaNo ratings yet
- Fuel Processing Technology: Ganesh L. Maddikeri, Aniruddha B. Pandit, Parag R. GogateDocument9 pagesFuel Processing Technology: Ganesh L. Maddikeri, Aniruddha B. Pandit, Parag R. GogatesureshNo ratings yet
- Biodiesel Production via Transesterification of TriglyceridesDocument2 pagesBiodiesel Production via Transesterification of TriglyceridesGhimis Simona BiancaNo ratings yet
- PDFDocument202 pagesPDFGhimis Simona BiancaNo ratings yet
- Measuring Nanoparticles with Light ScatteringDocument14 pagesMeasuring Nanoparticles with Light ScatteringGhimis Simona BiancaNo ratings yet
- Yi Wei, Jijian Hong, Weirong Ji T: Full Length ArticleDocument6 pagesYi Wei, Jijian Hong, Weirong Ji T: Full Length ArticleGhimis Simona BiancaNo ratings yet
- A Glycerol-Free Process To Produce Biodiesel by Supercritical Methyl Acetate Technology An Optimization Study Via Response Surface MethodologyDocument5 pagesA Glycerol-Free Process To Produce Biodiesel by Supercritical Methyl Acetate Technology An Optimization Study Via Response Surface MethodologyEsnaider Romero ArtetaNo ratings yet
- Methanol boosts sunflower oil interesterificationDocument4 pagesMethanol boosts sunflower oil interesterificationGhimis Simona BiancaNo ratings yet
- 4fdd PDFDocument7 pages4fdd PDFGhimis Simona BiancaNo ratings yet
- Bioresource Technology 294 (2019) 122097Document8 pagesBioresource Technology 294 (2019) 122097Ghimis Simona BiancaNo ratings yet
- Continuous HTL of Biomass Yields Biocrude FuelDocument10 pagesContinuous HTL of Biomass Yields Biocrude FuelGhimis Simona BiancaNo ratings yet
- Crossmark: Renewable and Sustainable Energy ReviewsDocument13 pagesCrossmark: Renewable and Sustainable Energy ReviewsGhimis Simona BiancaNo ratings yet
- Energy Conversion and ManagementDocument9 pagesEnergy Conversion and ManagementGhimis Simona BiancaNo ratings yet
- 25 - Current, Resistance, and Electromotive Force - R K ParidaDocument11 pages25 - Current, Resistance, and Electromotive Force - R K ParidaMonicaNo ratings yet
- Horizontal & Vertical Eccentricities in Mass and Stiffness DistributionDocument23 pagesHorizontal & Vertical Eccentricities in Mass and Stiffness Distributionmuktha mukuNo ratings yet
- Primera Ley Sistemas CerradosDocument68 pagesPrimera Ley Sistemas CerradospimpollompNo ratings yet
- Zirconia As A Dental BiomaterialDocument14 pagesZirconia As A Dental BiomaterialaliNo ratings yet
- DAB 2018 Componenets CatalogueDocument20 pagesDAB 2018 Componenets CataloguegobilgobilNo ratings yet
- Lab 5 - Analysis of Deflection Under Transverse Loading (Simply Supported Beam)Document8 pagesLab 5 - Analysis of Deflection Under Transverse Loading (Simply Supported Beam)Mohib ShareefNo ratings yet
- Chiller Carrier 30XA-400Document26 pagesChiller Carrier 30XA-400EdwinRamirezNo ratings yet
- SPE-181057-MS Novel Solutions and Interpretation Methods For Transient, Sandface Temperature in Vertical, Dry Gas Producing WellsDocument15 pagesSPE-181057-MS Novel Solutions and Interpretation Methods For Transient, Sandface Temperature in Vertical, Dry Gas Producing Wellsatilio martinezNo ratings yet
- RSXYP16 30KJY1 SiE00 07 Part 1 - Service Manuals - EnglishDocument193 pagesRSXYP16 30KJY1 SiE00 07 Part 1 - Service Manuals - EnglishghenceaNo ratings yet
- Bend Testing of Material For Ductility: Standard Test Methods ForDocument10 pagesBend Testing of Material For Ductility: Standard Test Methods ForSyafiq Irsyadillah JafarNo ratings yet
- Water Labs FullDocument4 pagesWater Labs FulljohnosborneNo ratings yet
- SRI CHAITANYA EDUCATIONAL INSTITUTIONS JR AIIMS S60 NEET-2024 SCHEDULEDocument5 pagesSRI CHAITANYA EDUCATIONAL INSTITUTIONS JR AIIMS S60 NEET-2024 SCHEDULEteen's teamNo ratings yet
- Machine Elements Tutorial on Screw Design and EfficiencyDocument2 pagesMachine Elements Tutorial on Screw Design and EfficiencydvarsastryNo ratings yet
- Training Plan Final - Leonardo FINALDocument2 pagesTraining Plan Final - Leonardo FINALCarl Roger AnimaNo ratings yet
- Presentation VDocument51 pagesPresentation VGagan SrivastavaNo ratings yet
- Overhead Transmission Line CapacitanceDocument20 pagesOverhead Transmission Line CapacitanceKyla BelgadoNo ratings yet
- Problem Sheet - 2 PDFDocument2 pagesProblem Sheet - 2 PDFJyotirmoy DekaNo ratings yet
- Minimum Vs Average Wall Tubes & Why It Matters - Elliott ToolDocument3 pagesMinimum Vs Average Wall Tubes & Why It Matters - Elliott ToolErsonNo ratings yet
- Unit-1: Mode Theory of Circular WaveguideDocument16 pagesUnit-1: Mode Theory of Circular WaveguideRahul KumarNo ratings yet
- Current Concepts: Biomechanics of Knee LigamentsDocument11 pagesCurrent Concepts: Biomechanics of Knee LigamentsBarbaraAndradeQuirozNo ratings yet
- Physics Paper 2 03 - 08Document78 pagesPhysics Paper 2 03 - 08Ho Fung ChernNo ratings yet