Professional Documents
Culture Documents
Lecture 1
Uploaded by
Ankan PalCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lecture 1
Uploaded by
Ankan PalCopyright:
Available Formats
8.
04 Quantum Physics
Lecture I
Lecturer
Vladan Vuletic
Available information
course policies announcements & suggested reading problem sets/solutions, practice exams student grades
Problem sets
posted by Thursday due following Thursday afternoon late homework not accepted (solutions are published online)
one lowest homework score will be dropped when calculating grades
Grading
Exam 1 20% Exam 2 20% Final 40% Problem sets 20% Collaboration on problem sets encouraged, but everybody has to submit own solution.
Textbooks
Gasiorowicz: required French & Taylor: strongly recommended Feynman, Lectures on Physics: selected chapters Texts as reference & reading as preparation. Lectures are basis; notes will be posted. Massachusetts Institute of Technology I-1
8.04 Quantum Physics
Lecture I
Learning goals for 8.04
boundary between classical and quantum physics understand crucial experiments that paved way for development of quantum mechan ics understand & interiorize probability amplitude and interference concepts that are at the heart of QM single-particle quantum mechanics for external degrees of freedom; Schr odinger equation internal degrees of freedom; e.g., spin: 8.05; many body quantum physics: 8.06 and beyond some formal structure of QM (operators, expectation values, commutators, Dirac notation) further development: 8.05 understand interface between mathematical structure (Schr odinger equation as partial dierential equation) and physical interpretation, measurement, uncertainty, correla tions, and entanglement study important QM systems: harmonic oscillator, hydrogen atom At the end of this course you should be able to: - solve simple QM single-particle problems in one and three dimensions (scat tering, tunneling, bound states) - give a physical interpretation of mathematical entities (operators, wavefunc tion, state representation in dierent bases, Fourier transform, Heisenberg un certainty relation) - appreciate & understand the all-importance of interference eect (addition of probability amplitudes) in QM 8.04: only non-relativistic QM
Problems with/failures of classical mechanics (CM)
CM fails at microscopic level CM cannot explain, e.g.,
- stability of individual atoms
Massachusetts Institute of Technology I-2
8.04 Quantum Physics - emission spectra of atoms
- molecular bonds
- chemical properties, chemical reactions
- properties of solids
Lecture I
Predictions from CM contradict some experimental facts in thermodynamics: - blackbody spectrum (spectral density of thermal electromagnetic radiation) - heat capacity of a gas of diatomic molecules
Blackbody spectrum
Classical thermodynamics predicts that each degree of freedom at absolute temperature T carries, on average, an energy 1 k T. 2 B kB = 1.38 1023 J/K (the Boltzmann constant) (1-1)
Each electromagnetic mode constitutes a degree of freedom. In a container with perfectly
Figure I: Metal container with discrete electromagnetic modes due to boundary conditions on the
walls.
Massachusetts Institute of Technology
I-3
8.04 Quantum Physics
Lecture I
conducting walls the modes satisfy n = 2nL , n 1 integer. There are innitely many shortwavelength modes inside the container. If each contains average energy kB T , then the energy stored inside the container must be innite. QM. The mode frequency n = cn sets a natural energy scale (photon energy) En = hn , (h = 6.6 1034 Js is Plancks constant), modes whose natural energy scale En is much larger that kB T are not thermally populated, they remain empty and carry no thermal energy. High-energy modes with En kB T are frozen out. They do not carry thermal energy. Energy inside box remains nite, spectrum and energy per mode agree with experi ments. (Planck formula: 8.044).
Heat capacity of diatomic gas
Monatomic gas of N atoms has heat capacity (energy stored at temperature T ) given by CV = 3 NkB , in agreement with measurements. There are three translational degrees of 2 freedom per atom, each degree of freedom stores kinetic energy 1 k T. 2 B For a gas of N diatomic molecules, we expect CV = 7 Nk , 2 N atoms with translational B 2 degrees of freedom, or 3 center-of-mass translational degrees of freedom, 2 rotational de grees of freedom, 2 vibrational degrees of freedom (one kinetic energy, one potential en ergy). However, observation at room temperature is CV = 5 NkB . 2
(a) One vibrational degree of freedom.
(b) Two rotational degrees of freedom.
Figure II: Degrees of freedom of a diatomic molecule. Explanation. Vibrational mode with frequency has natural energy scale E = h kB T , is frozen out, does not contribute to heat capacity at room temperature. At high temperature kB T h : CV 7 NkB . 2 What about electronic degrees of freedom inside atom? Also frozen out. En 1 eV kB T =
1 40
eV at room temperature.
Massachusetts Institute of Technology
I-4
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5782)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Dialectics of Seeing - Buck-MorssDocument252 pagesThe Dialectics of Seeing - Buck-Morsselvisthecat100% (3)
- Fakulteti Juridik Universiteti I Prishtinës "Hasan Prishtina"Document2 pagesFakulteti Juridik Universiteti I Prishtinës "Hasan Prishtina"Jonida ElshaniNo ratings yet
- Rizal Position PaperDocument3 pagesRizal Position PaperAndrea ColumbanoNo ratings yet
- Synopsis of Philippine HistoryDocument60 pagesSynopsis of Philippine HistoryCathirine OliverNo ratings yet
- Application Form: Membership of Pakistan - (Country Name)Document2 pagesApplication Form: Membership of Pakistan - (Country Name)Muhammad ShahzaibNo ratings yet
- Gender Spectrum - My Gender JourneyDocument3 pagesGender Spectrum - My Gender JourneyGender SpectrumNo ratings yet
- Research On HIBADocument9 pagesResearch On HIBAPixel GeekNo ratings yet
- Precolonial Vs 20th CenturyDocument1 pagePrecolonial Vs 20th CenturyLance BoritoNo ratings yet
- Mozart and The EnlightenmentDocument37 pagesMozart and The EnlightenmentwtempleNo ratings yet
- Proposed Schedule For The Utilization of MELCs - HUMSSDocument22 pagesProposed Schedule For The Utilization of MELCs - HUMSSLorelie Dioso HonestoNo ratings yet
- Bha Wal PurDocument26 pagesBha Wal Purاسامہ اقبالNo ratings yet
- Unit 6Document8 pagesUnit 6Nguyen Cuong75% (4)
- Political Integration of IndiaDocument2 pagesPolitical Integration of Indiaanahata2014No ratings yet
- Indonesian Law on Narcotics Crimes and PunishmentsDocument2 pagesIndonesian Law on Narcotics Crimes and PunishmentsAsnu FayakunNo ratings yet
- Cuba LibreDocument321 pagesCuba LibreAvijith Chandramouli100% (1)
- Sangguinian Vs Punong BrgyDocument3 pagesSangguinian Vs Punong BrgyVictor FaeldonNo ratings yet
- American Outdoor Brands Report To Shareholders On Gun SafetyDocument26 pagesAmerican Outdoor Brands Report To Shareholders On Gun SafetyJim KinneyNo ratings yet
- The IlluminatiDocument2 pagesThe IlluminatiLisa OdehNo ratings yet
- The Peacock Chair by Mona AlcudiaDocument52 pagesThe Peacock Chair by Mona AlcudiaMona Alcudia100% (3)
- The Emergence of Nation State SystemDocument9 pagesThe Emergence of Nation State SystemEraj NoumanNo ratings yet
- Panizo Mayo, 1Document4 pagesPanizo Mayo, 1dfmrodNo ratings yet
- Edward Shils 1957 Primordial Personal Sacred and Civil TiesDocument21 pagesEdward Shils 1957 Primordial Personal Sacred and Civil TiesPrafulla NathNo ratings yet
- Prime Minister Shocked Over Huge Assets of KarunanidhiDocument19 pagesPrime Minister Shocked Over Huge Assets of KarunanidhiArun KumarNo ratings yet
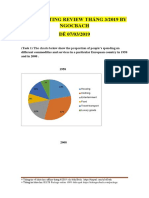
- Ielts Writing Review Thang 3 - 2019 by Ngocbach PDFDocument24 pagesIelts Writing Review Thang 3 - 2019 by Ngocbach PDFMinh KhuêNo ratings yet
- What Is Halalan ToyyibanDocument2 pagesWhat Is Halalan ToyyibanYazlin Yusof100% (2)
- R. W. Davies, Oleg V. Khlevnyuk, Stephen G. Wheatcroft (Auth.) - The Industrialisation of Soviet Russia 6 - The Years of Progress - The Soviet Economy, 1934-1936 (2014)Document513 pagesR. W. Davies, Oleg V. Khlevnyuk, Stephen G. Wheatcroft (Auth.) - The Industrialisation of Soviet Russia 6 - The Years of Progress - The Soviet Economy, 1934-1936 (2014)thoranllares100% (1)
- Poum Spanishc.w.Document21 pagesPoum Spanishc.w.Piero PaddinoNo ratings yet
- A Grin Without A CatDocument5 pagesA Grin Without A CatlukuminaNo ratings yet
- Toni MorrisonDocument6 pagesToni MorrisonScott M B GustafsonNo ratings yet
- Carta de Las Naciones Unidas ORIGINALDocument188 pagesCarta de Las Naciones Unidas ORIGINALdipublicoNo ratings yet