Professional Documents
Culture Documents
Phosphorus Cycle
Uploaded by
Astrid Harfera PassadanaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Phosphorus Cycle
Uploaded by
Astrid Harfera PassadanaCopyright:
Available Formats
Phosphorus cycle
http://en.wikipedia.org/wiki/Phosphorus_cycle From Wikipedia, the free encyclopedia
Jump to: navigation, search This article may require cleanup to meet Wikipedia's quality standards.
Please improve this article if you can. (October 2008)
The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movements of phosphorus, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth.
[edit] Phosphorus in the environment
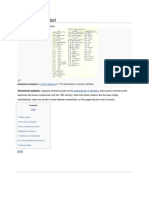
The aquatic phosphorus cycle Phosphorus is an essential nutrient for plants and animals in the form of ions PO43- and HPO42- . It is a part of DNA-molecules and RNA-molecules, molecules that store energy (ATP and ADP) and of fats of cell membranes. When Phosphorus is in a compound (phosphate), it plays an important role in holding the DNA and RNA together.[1] Phosphorus is also a building block of certain parts of the human and animal body, such as the bones and teeth. Phosphorus forms parts of important life sustaining molecules but is not very common in the biosphere. Phosphorus does not enter the atmosphere, remaining mostly on land and in rock and soil minerals. 80 percent of the phosphorus is used to make fertilizers and a type of phosphorus such as dilute phosphoric acid is used in soft drinks. Phosphates may be effective in such ways but they also cause pollution problems in lakes and streams. Over enrichment of phosphate can lead to algal bloom, because of the excess of nutrients. This causes more algae to grow, bacteria consumes the algae and causes more bacteria to increase in numbers. They use all the oxygen in the water during cellular respiration, causing many fish to die.
Phosphorus normally occurs in nature as part of a phosphate ion, consisting of a phosphorus atom and some number of oxygen atoms, the most abundant form (called orthophosphate) having four oxygens: PO43-. Most phosphates are found as salts in ocean sediments or in rocks. Over time, geologic processes can bring ocean sediments to land, and weathering will carry terrestrial . Plants absorb phosphates from the soil, then bind the phosphate into organic compounds. The plants may then be consumed by herbivores who in turn may be consumed by carnivores. After death, the animal or plant decays, and the phosphates are returned to the soil. Runoff may carry them back to the ocean or they may be reincorporated into rock. The primary biological importance of phosphates is as a component of nucleotides, which serve as energy storage within cells (ATP) or when linked together, form the nucleic acids DNA and RNA. Phosphorus is also found in bones, whose strength is derived from calcium phosphate, and in phospholipids (found in all biological membranes). Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles. Unlike other cycles of matter compounds, phosphorus cannot be found in air as a gas. This is because at normal temperature and circumstances, it is a solid in the form of red and white phosphorus. It usually cycles through water, soil and sediments. Phosphorus is typically the limiting nutrient found in streams, lakes and fresh water environments. As rocks and sediments gradually wear down, phosphate is released. In the atmosphere phosphorus is mainly small dust particles. Initially, phosphate weathers from rocks. The small losses in a terrestrial system caused by leaching through the action of rain are balanced in the gains from weathering rocks. In soil, phosphate is absorbed on clay surfaces and organic matter particles and becomes incorporated (immobilized). Plants dissolve ionized forms of phosphate. Herbivores obtain phosphorus by eating plants, and carnivores by eating herbivores. Herbivores and carnivores excrete phosphorus as a waste product in urine and feces. Phosphorus is released back to the soil when plants or animal matter decomposes and the cycle repeats.
[edit] References
1. ^ http://www.enviroliteracy.org/article.php/480.html
Part III of "Matter cycles": The phosphorus cycle, Lenntech Water treatment & air purification, Holding B.V. 2006 Environmental Literacy Council - Phosphorus Cycle Monitoring and assessing water quality, section 5.6 Phosphorus - EPA Prentice Hall Biology, Kenneth R. Miller and Joseph Levine 2001 Katie Corbin-Soil Microbiology
[1]
Biogeochemical cycles Carbon cycle - Hydrogen cycle - Nitrogen cycle Oxygen cycle - Phosphorus cycle - Sulfur cycle - Water cycle
Retrieved from "http://en.wikipedia.org/wiki/Phosphorus_cycle" Categories: Ecology | Soil biology | Soil chemistry | Biogeography Hidden categories: Cleanup from October 2008 | All pages needing cleanup
You might also like
- MetodeDocument3 pagesMetodeAstrid Harfera PassadanaNo ratings yet
- Jadwal Semester Gasal 2013-2014 S1 Kimia Versi Terbaru (19 Juli 2013)Document10 pagesJadwal Semester Gasal 2013-2014 S1 Kimia Versi Terbaru (19 Juli 2013)Faisal Rizki GumelarNo ratings yet
- Water CycleDocument7 pagesWater CycleAstrid Harfera PassadanaNo ratings yet
- OzoneDocument20 pagesOzoneAstrid Harfera PassadanaNo ratings yet
- Oxygen CycleDocument3 pagesOxygen CycleAstrid Harfera PassadanaNo ratings yet
- Sulfur CycleDocument2 pagesSulfur CycleAstrid Harfera PassadanaNo ratings yet
- Hydrogen CycleDocument2 pagesHydrogen CycleAstrid Harfera PassadanaNo ratings yet
- Carbon CycleDocument7 pagesCarbon CycleAstrid Harfera PassadanaNo ratings yet
- Nitrogen CycleDocument6 pagesNitrogen CycleAstrid Harfera PassadanaNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Alternadores GPW Gpa GPF GSW Gsa GSF en 02Document39 pagesAlternadores GPW Gpa GPF GSW Gsa GSF en 02Gesiel SoaresNo ratings yet
- LPG PropertiesDocument2 pagesLPG Propertiesvvk557No ratings yet
- Batch ReactorDocument4 pagesBatch ReactorHarini BugattiveyronNo ratings yet
- Daily Lesson Log TemplateDocument64 pagesDaily Lesson Log TemplateArlene ChavezNo ratings yet
- EPA Corrosion ManualDocument141 pagesEPA Corrosion Manualnert100% (1)
- Vapor Phase Soldering TechniqueDocument5 pagesVapor Phase Soldering TechniquealisakeerpkNo ratings yet
- Copernican PrincipleDocument7 pagesCopernican Principlemaddy555No ratings yet
- TRANSIENT HEAT TRANSFER CALCULATIONSDocument3 pagesTRANSIENT HEAT TRANSFER CALCULATIONSSanith RenjalNo ratings yet
- CemCRETE Internal Presentation Apr 2004Document42 pagesCemCRETE Internal Presentation Apr 2004Muhammad ImranNo ratings yet
- Flowmetter KytolaDocument4 pagesFlowmetter Kytolason tran lamNo ratings yet
- Sensors 3Document59 pagesSensors 3zubairawNo ratings yet
- Refractory Datasheet 2 - KS-4V PLUSDocument2 pagesRefractory Datasheet 2 - KS-4V PLUSSubrata DasNo ratings yet
- Extended Impregnation Kraft Cooking of SoftwoodDocument50 pagesExtended Impregnation Kraft Cooking of SoftwoodMoksadur RahmanNo ratings yet
- Catalog Industrial Elevator BucketsDocument36 pagesCatalog Industrial Elevator BucketsKoray OzturkNo ratings yet
- TrimyristinDocument3 pagesTrimyristindinna_dinunNo ratings yet
- Laser Parameters PDFDocument20 pagesLaser Parameters PDFayyappa laserNo ratings yet
- SDB 7533 Ie enDocument12 pagesSDB 7533 Ie enDavid G. VegaNo ratings yet
- Biological MoleculesDocument98 pagesBiological MoleculesSuyashNo ratings yet
- Lidocaine Base and Hydrochloride: Groningsson, Lindgren, Lundberg, SandbergDocument37 pagesLidocaine Base and Hydrochloride: Groningsson, Lindgren, Lundberg, SandbergtikaNo ratings yet
- Freeze-Drying Process Development For Protein PharmaceuticalsDocument23 pagesFreeze-Drying Process Development For Protein Pharmaceuticalsboddarambabu100% (1)
- Industrial FRP Tank Water Media FilterDocument4 pagesIndustrial FRP Tank Water Media FilterT Nagaraju AF VetaranNo ratings yet
- Course: Department Specific Elective 2-: HPHDS5021T Nuclear and Particle PhysicsDocument3 pagesCourse: Department Specific Elective 2-: HPHDS5021T Nuclear and Particle PhysicsGrim Reaper Kuro OnihimeNo ratings yet
- Slug Catcher Conceptual DesignDocument8 pagesSlug Catcher Conceptual Designfanziskus100% (1)
- Austenitic Cast Iron Welding Detailsfrequently Aske-WPS OfficeDocument17 pagesAustenitic Cast Iron Welding Detailsfrequently Aske-WPS Officearjun prajapatiNo ratings yet
- Thermal Control of High Power Applications On Cubesats: October 2018Document16 pagesThermal Control of High Power Applications On Cubesats: October 2018Josue Manuel Pareja ContrerasNo ratings yet
- Ionic EquilibriumDocument91 pagesIonic EquilibriumGabrielNo ratings yet
- Alchemical SymbolDocument6 pagesAlchemical SymbolMilind Goel ReplexNo ratings yet
- Asam Mefenamat EmulgelDocument5 pagesAsam Mefenamat EmulgelVi Vian HiuNo ratings yet
- Chapter 16 Halogen DerivativesDocument11 pagesChapter 16 Halogen DerivativesSabina SabaNo ratings yet
- Dyes and Pigments: SciencedirectDocument9 pagesDyes and Pigments: SciencedirectrishabhNo ratings yet