Professional Documents
Culture Documents
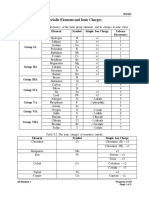
Chemistry Periodic Table
Uploaded by
api-2633229040 ratings0% found this document useful (0 votes)
13 views3 pagesOriginal Title
chemistry periodic table
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
13 views3 pagesChemistry Periodic Table
Uploaded by
api-263322904Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
The Periodic Table
History of the Idea of Elements
Ancient Greeks
the basic substances from which other materials are made
4 elements: earth, air, re, water
Boyle
rst reasonably accurate defn.
a substance which cannot be broken down into simpler materials by chemical
means.
compounds: combinations of elements which can be formed from and broken
down into these elements.
Davy
new electrochemical techniques for breaking compounds down into elements.
electrolysed a moist solid sample of potassium hydroxide producing small pieces
of a shining metal (potassium) which rapidly burnt with a lilac ame.
sodium form sodium hydroxide in a similar manner
also isolated barium, strontium, calcium and magnesium
Moseley
discovered a characteristic positive charge in the atomic nucleus of each element
called this charge the atomic number
advancement of defn: a substance all of whose atoms have the same atomic
number
What is the periodic table?
It is a list of elements arranged so as to demonstrate trends in their physical and
chemical properties.
History of the Periodic Table
Dobereiners Triads
groups of three elements
similar chemical properties
atomic weight of the second is midway between that of the rst and the third
example: Li, Na, K
restricted to a small number of elements
Newlands Octaves
when in order of atomic weight, properties repeat every eight elements
worked for rst 17 elements
forced all known elements - Cu & Ag with Li, Na, K
principle: periodic reoccurrence of properties when elements are arranged in order
of increasing atomic weight.
Mendeleevs Sub-Groups
principle: periodic reoccurrence of properties when elements are arranged in order
of increasing atomic weight.
in order of increasing atomic weight
listed separately elements who did not t in with the properties of the main groups
- Cu & Ag.
I and Te out of correct order because of their properties
left gaps which represented elements not yet discovered
choosing 3 gaps, he predicted the properties of the elements
Moseley
discovery of characteristic positive charge / atomic number
table in order of increasing atomic number
Compare Mendeleevs and the modern periodic table.
Mendeleevs Modern
inc. atomic weight (mass) inc. atomic number
reversed some pairs (Te & I) no reversed pairs
left gaps for undiscovered elements gaps lled
no noble gases noble gases present
fewer elements more elements
did not put transition elements /
lanthanides / actinides in separate block
transitions / lanthanides / actinides in
separate block
The Alkali Metals
increasing reactivity going down the group
soft metals - can be cut with a knife
low densities
shiny but tarnish quickly
sodium + oxygen sodium oxide
react vigorously with water to form basic soln. and hydrogen
sodium + water sodium hydroxide + hydrogen
The Alkaline Earth Metals
increasing reactivity going down the group
harder than the alkali metals
less reactive than their corresponding alkali metals
react with water but less vigorously than their corresponding alkali metals
calcium + water calcium hydroxide + hydrogen
The Halogens
nonmetals
decreasing reactivity going down the group
low melting/boiling points
react with hydrogen to form acidic soln.s
hydrogen + chlorine
react vigorously with alkali metals forming white salts
sodium + chlorine sodium chloride
The Noble Gases
all gases at room temp.
boiling point and density increase going down the group
least reactive
You might also like
- 28 02 14 The Exchange System in PlantsDocument53 pages28 02 14 The Exchange System in Plantsapi-263322904No ratings yet
- Revision Book For Senior BiologyDocument85 pagesRevision Book For Senior Biologyapi-263322904No ratings yet
- Biology Focus Book QsDocument196 pagesBiology Focus Book Qsapi-263322904No ratings yet
- Chemistry Notes Gas LawsDocument5 pagesChemistry Notes Gas Lawsapi-263322904100% (1)
- 2 2 3 EnzymesDocument49 pages2 2 3 Enzymesapi-263322904No ratings yet
- HL Titration QuestionsDocument8 pagesHL Titration Questionsapi-263322904No ratings yet
- 4 A Titration Procedures SummaryDocument6 pages4 A Titration Procedures Summaryapi-263322904No ratings yet
- Higher L BacteriaDocument25 pagesHigher L Bacteriaapi-263322904No ratings yet
- 1 3 Nutrition Revision A1 PosterDocument2 pages1 3 Nutrition Revision A1 Posterapi-263322904No ratings yet
- 3 1 6 AmoebaDocument32 pages3 1 6 Amoebaapi-263322904No ratings yet
- FungiDocument54 pagesFungiapi-263322904No ratings yet
- Leaving Cert EnglishDocument4 pagesLeaving Cert Englishapi-263322904No ratings yet
- 3 3 1 Nutrition in The PlantDocument15 pages3 3 1 Nutrition in The Plantapi-263322904No ratings yet
- Flowering Plants Structureud 06021014Document52 pagesFlowering Plants Structureud 06021014api-263322904No ratings yet
- 3 4 4 Lungs BreathingDocument39 pages3 4 4 Lungs Breathingapi-263322904No ratings yet
- Acquainted With The NightDocument3 pagesAcquainted With The Nightapi-263322904No ratings yet
- 1 Blood Cells ClassDocument27 pages1 Blood Cells Classapi-263322904No ratings yet
- 1 Male Structure and HormonesDocument39 pages1 Male Structure and Hormonesapi-263322904No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5782)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Astm F467MDocument9 pagesAstm F467Mhouk sukNo ratings yet
- LGC StandardsDocument5 pagesLGC StandardsJacekNo ratings yet
- BQ Subcon - MRT Sungai BulohDocument8 pagesBQ Subcon - MRT Sungai BulohChiaChiNo ratings yet
- Vitamins & Minerals (List of Students)Document3 pagesVitamins & Minerals (List of Students)Alex DincaNo ratings yet
- Types of Chemical ReactionsDocument4 pagesTypes of Chemical ReactionsHannah Adriene LavillesNo ratings yet
- Anodasi Alumunium PDFDocument8 pagesAnodasi Alumunium PDFRisa HapsariNo ratings yet
- Mini Interruptores Termomagnéticos e Interruptores DiferencialesDocument52 pagesMini Interruptores Termomagnéticos e Interruptores DiferencialesJay SantanNo ratings yet
- Bagan Kation 1 - 3Document7 pagesBagan Kation 1 - 3Angelica ErnitaNo ratings yet
- IGCSE Chemistry Tests For IonsDocument1 pageIGCSE Chemistry Tests For Ionscharliemason007No ratings yet
- CHEMICAL BONDING TYPESDocument5 pagesCHEMICAL BONDING TYPESzafarchem_iqbalNo ratings yet
- Naming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights ReservedDocument5 pagesNaming Chemical Compounds Worksheet: © 2004 Cavalcade Publishing, All Rights Reservedpao manaligodNo ratings yet
- QC ProbsDocument2 pagesQC ProbsFaith de Leon ♥No ratings yet
- 1617 Level M Chemistry Topic 12 Salt Preparation BQ-2Document4 pages1617 Level M Chemistry Topic 12 Salt Preparation BQ-2MikhaelNo ratings yet
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Periodic TableDocument2 pagesPeriodic TableNur NajwaNo ratings yet
- Food ChemistryDocument21 pagesFood Chemistrylinm@kilvington.vic.edu.auNo ratings yet
- Group 1 (Alkali Metals) - Lithium, Sodium, Potassium QPDocument8 pagesGroup 1 (Alkali Metals) - Lithium, Sodium, Potassium QPRODGERS BANDANo ratings yet
- 9701 m19 QP 33 PDFDocument12 pages9701 m19 QP 33 PDFAbubakar shaban omarNo ratings yet
- Chemical formulas and synonymsDocument30 pagesChemical formulas and synonymsMariaFrancescaBiancoSaenzNo ratings yet
- Elements & Periodic Table GuideDocument9 pagesElements & Periodic Table GuideMaMtNo ratings yet
- Reactivity Series ExperimentDocument3 pagesReactivity Series ExperimentSourabh DasNo ratings yet
- Chemistry Form 6 Sem 2 04 Notes STPM 2014/2013Document27 pagesChemistry Form 6 Sem 2 04 Notes STPM 2014/2013Raj Nittiya SugumaranNo ratings yet
- ASTM B348-06aStandardSpecificationforTitaniumandTitaniumAlloyBarsandBillets PDFDocument8 pagesASTM B348-06aStandardSpecificationforTitaniumandTitaniumAlloyBarsandBillets PDFtahirabbasNo ratings yet
- Naming Ionic Compounds WKSHT #1 PDFDocument4 pagesNaming Ionic Compounds WKSHT #1 PDFZer Min SimNo ratings yet
- 2007 Effect of Alloying Elements and Tarnishing EffectsDocument15 pages2007 Effect of Alloying Elements and Tarnishing EffectsmarcosNo ratings yet
- O Final Mj14 Chemistry P 1 5070 01Document373 pagesO Final Mj14 Chemistry P 1 5070 01AHMADNo ratings yet
- Material Name C MN Si CR Mo Ni N CB Cu NB: Filler Metal 1 Chemical CompositionDocument5 pagesMaterial Name C MN Si CR Mo Ni N CB Cu NB: Filler Metal 1 Chemical CompositionGerard van BrakelNo ratings yet
- Inox Tester User Manual EP04Document36 pagesInox Tester User Manual EP04Ramon PachecoNo ratings yet
- General Values For ChemicalsDocument9 pagesGeneral Values For ChemicalsAakash SharmaNo ratings yet
- GB 17107Document20 pagesGB 17107Qiuo ShenNo ratings yet