Professional Documents
Culture Documents
Production of Polyethylene
Uploaded by
tokiinCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Production of Polyethylene
Uploaded by
tokiinCopyright:
Available Formats
High Density Polyethylene
HDPE stands for High Density Polyethylene. Polyethylene can be produced by the
polymerization reaction of ethylene. There are several grades of polyethylene are available in
market such as LDPE, LLDPE, MDPE, HDPE, UHMWPE, etc. HDPE is a very useful form
polyethylene, which is used most extensively through out the world.
Polyethylene is normally classified on the basis of its density. The range of density for
HDPE is 0.95-0.96 kg/dm
3
. The properties of all these grades strongly depend upon on density.
So HDPE is preffered on other grades for most
applications
At the very close of the 19
th
century, German chemist Hans von Pechmann noted a
precipitate while working with a form of methane in ether. In 1900, German chemist Eugen
Bamberger and Friedrich Tschirner identified this compound as polyethylene, a very close
cousin to polyethylene. Thirty year later, an American Chemist created a high-density residue by
subjecting ethylene to a large amount of pressure.
History of HDPE
Working with ethylene at high pressure, British chemists Eric Fawcett and Reginald Gibson
created a solid form of polyethylene in 1935. Its first commercial application came during World
War II, when the British used it to insulate radar cables. Up until the 1950s the only type of
polyethylene produced was low-density polyethylene. Low Density polyethylene was being
produced at extremely high pressures. This high-pressure polymerization created polyethylene
with many branches; the branches are created due to intermolecular and intermolecular chain
transfer during polymerization. The mechanism for the polymerization of low-density
polyethylene is free radical polymerization. The uses of low-density polyethylene are limited due
to high number of branches. Because of the extreme pressure needed to create low-density
polyethylene and its limited uses, Karl Ziegler was trying to create polyethylene at atmospheric
pressure. Karl Ziegler, a German scientist, made the greatest contribution to producing high-
density polyethylene.
(18)
In 1953, Karl Ziegler and Erhard Holzkamp invented high-density polyethylene (HDPE). The
process included the use of catalysts and low pressure, which is the basis for the formulation of
many varieties of polyethylene compounds. Two years later, in 1955. HDPE was produced as
pipe. For his successful invention of HDPE, Zeigler was awarded the 1963 Nobel Prize for
chemistry.
Properties of HDPE
High-density polyethylene (HDPE) is a thermoplastic material composed of
carbon and hydrogen atoms joined together forming high molecular products. Methane gas is
first converted into ethylene, then, with the application of heat and pressure into polyethylene.
The polymer chain may be 500,000 to 1,000,000 carbon units long. Short and long side chain
molecules exist with the polymers long chain molecules. The longer the main chain, the greater
the number of atoms, and consequently, greater the molecular weight. The molecular weight,
molecular weight distribution and the amount of branching determine many of the mechanical
and chemical properties of the end product.
(17)
High-density polyethylene resin has a greater proportion of crystalline regions
than low-density polyethylene. The size and size distribution of crystalline regions are
determinants of the tensile strength and environmental stress crack resistance of the end
products. HDPE, with fewer branches than LDPE, has a greater proportion of crystals, which
results in greater density and greater strength. So HDPE would be too brittle to be functional and
upon heating, the ordered crystalline structure regresses to the disordered amorphous state, with
cooling, the partially crystalline structure is recovered.
Since HDPE is a thermoplastic material, the molecular chains are not cross-linked and
such plastics melt with application of a sufficient amount of heat. So with the application of heat,
it can be shaped, formed, molded or extruded.
Structure of HDPE is shown below
Uses Of HDPE
HDPE is mainly used for pipes and pipe fittings for water transportation. Toys, bowls,
buckets, milk bottles, crates, tanks, containers. Film for packaging. Blown bottles for food.
MDPE (Medium Density Polyethylene) is used for gas pipes and fittings.
For environmental protection, HDPE Geomembranes are the perfect solution for lining
landfill facilities. Field Lining Systems, Inc., has extensive experience installing many types of
geomembranes in a variety of landfill applications. Landfill lining today takes a great deal of
expertise and planning to ensure coordination with other contractors involved in the installation
itself.
It has proven itself, by the millions of square feet installed, that they have the
knowledge, professionalism and capabilities to ensure a top quality lining system.
Landfill covers pose some difficulty due to the constant settling and shifting of the decomposing
refuse heaps, but there are HDPE membranes that have been used for this purpose.
(17)
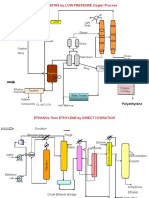
Manufacturing Techniques:
Two main polymerization processes can produce HDPE
(7)
1. Co-ordination Polymerization
2. Addition Polymerization
But most employed method of production of High Density Polyethylene is Co-ordination
Polymerization.
Co-ordination Polymerization carried out in following steps
i. Catalyst activation
ii. Initiation
iii. Propagation
iv. Termination
i. Catalyst Activation
(7)
TiCl
4
is a catalyst and (C
2
H
5
)
3
Al is co-catalyst. These both react with each other and produce
active site for initiation step. Reaction is give as
4 3 5 2 3 5 2 4
) ( ) ( AlCl H C T Al H C Cl T
i i
3 5 2
) ( H C T
i
is active site, which is used in initiation process?
ii. Initiation
Initiation is addition of the active site (primary radical) to the ethylene monomer. Where k
i
is the
rate constant for the initiation.
3 5 2 2 2 2 2 3 5 2
) ( ) ( H C CH CH T CH CH H C T
i i
iii. Propagation
Propagation involves the addition of further molecules to active end of the chain, which carries
an unpaired electron. Repetition of the propagation step leads to the information of a long chain
polymer with radial character. The mechanism is classified as anionic polymerization or "living"
polymerization. Living polymerization means the polymerization continues until the
concentration of ethylene runs out. Because of this, the molecular weight of the polyethylene
created can be extremely high.
(7)
3 5 2 1 2 2 2 2 3 5 2 2 2
) ( ) ( ) ( H C CH CH T CH nCH H C CH CH T
n i i
Where K
p
is the rate constant of the propagation reaction.
iv. Termination:
As propagation step continue, the molecular weight will also continually increased. One way
to control the molecular weight of high-density polyethylene created is through chain transfer
reagents. Some typical chain transfer reactions are shown below:
Chain Transfer with monomer:
This reaction occurs with some heterogeneous Ziegler-Natta catalysts.
Transfer with organometallic compounds:
Chain transfer with hydrogen
The reaction most important to controlling the molecular weight of high-density polyethylene
created by the Ziegler Process is chain transfer with the introduction of hydrogen. Where K
t
is
the rate of chain transfer.
Manufacturing process
The manufacturing process for polypropylene can be classified into three categories based on the
phase in which reaction takes place.
1. Gas Phase Polymerization
2. Slurry Phase Polymerization
3. Solution Phase Polymerization
Slurry Polymerization
There are many types of Slurry process, which are named on the name of companies, which
are producing HDPE. These processes are
(7)
i. Dupont Process
ii. Hoechst Process
iii. Mitsubishi Process
iv. Montelson Process
v. Phillips Process
vi. Solvay Process
In this type of process, polymerization of ethylene in suspension in a hydrocarbon
diluent is carried out. Diluent is a poorer solvent of polyethylene. Hence the polymer or
copolymer separates out from the diluent as fine particles. Hence, the viscosity of the diluent
does not increase as rapidly as in the solution process. As a result, a higher concentration of
polymer can be maintained in the reactors.
Some of the advantages of slurry-type processes include a higher volume yield of
product for a given size reactor, as well as the greater ease of diluent removal. On the other hand,
residence times are usually longer than in most solution processes. Still another advantage is the
potential for making powders, suitable for rotomoulding, directly in the reactor, thus cutting out
the expensive grinding step.
Reactors used for slurry processes may take any of several forms, from kettles to loop-
type designs. The latter have high surface-to volume ratios, which are advantageous for
controlling reactor temperature, so necessary in maintaining molecular weight and molecular
weight distribution as desired.
A disadvantage of the slurry type process is the greater difficulty of automation, since
there are fewer ways to immediately sense any changes in the product itself. Also, slurry
processes tend to make more 'twilight' material in switching from one grade to another. Also,
many processes are susceptible to fouling.
In practice, most slurry processes, unless modified, tend to yield very high molecular
weight materials, which are not commercially useful. Thus, a "chain stopper" or chain transfer
agent is often required. For Ziegler-type catalysts, hydrogen is generally used; it tends to give a
"clean" product and is not extensively expensive.
Because of their tendency to make higher molecular weights, most slurry processes
perform well in making blow molding grades; in some instances, however, tandem reactors may
be required for broad MWDs. Injection molding grades are harder o make.
Suspension polymerization, like the Hoechst-Ziegler technology, is still one of the most
mature, flexible, versatile and widely used processes. It is the only technology capable of
producing the wider range of HDPE polymers - and polymerization of ultra-high molecular
weight HDPE as well.
(7)
2.3.3 Solution Phase Polymerization
In solution processes, ethylene monomer and co-monomers are dissolved in hot
cyclohexane or other solvent suitable for polyethylene. Catalyst is introduced into the reactor and
the temperature maintained above 140-150C - the polyethylene melting point - at reactor
pressure. Polymerization ensues giving out large heat energy. Some processes use water-
jacketing to remove reaction heat, while in others cooling is done by monomer refrigeration.
(7)
Solution processes are generally run at moderately high pressures and temperatures and
require heavier wall reactors than other processes. However, because of the beneficial effect of
increased temperature on reaction rates, catalyst efficiencies are usually higher with short
residence times. This allows a higher production rate for a given size reactor.
Processes can be highly automated. For example, monitoring reaction parameters can
control product molecular weight, and reactor conditions can be changed via feedback loops.
This type of process is inherently limited in the amount of polymer, which can be kept in
solution: 35-40% is the absolute maximum. Also, making high molecular weight polymer gives
difficulties by putting high torque on the stirrer, dropping out of the solution as gel, and fouling
the reactor. Thus, it is more difficult to make extrusion blow molding grades of HDPE with
solution processes, particularly those requiring a very high molecular weight component for high
melt strength and die swell. On the other hand, solution processes generally excel in producing
injection-molding grades, where narrow MWDs and lower MWs are required.
(7)
2.4 Common Steps Involved in all Processes:
Catalyst formation and activation
Monomer purification (drying)
Polymerization reaction and addition of co-monomers
Flashing and separation of unrelated monomer and co monomers for recycle via a
compressor
Drying or purging of monomer (and solvents, if used) and Catalyst deactivation
Monomer and solvent recovery and purification by distillation
Addition and blending of additives with the polymer powder or granules
Melting, mixing, devolatilizing, melt filtering and palletizing in an extrusion line.
Bulk storage, blending, bulk loading and packaging.
Capacity of Plant:
The key to successful production of HDPE is the reaction. So we assume that the ethylene
gas, which is used as a main component for production of HDPE, is about 100% pure. The
reaction conditions are
Temperature = 80-90 C
o
Pressure = 8-10 atm
Residence time or Cycle time = 3.0 hrs
Overall Rate of reaction at these conditions = 98.5%
Capacity of plant = 150 TPD
HDPE powder = 6250 Kg/hr
Basis: 1 hour operation
Ethylene required = 6250 * 1.02
= 6375 Kg/hr
Catalyst = 0.0099 % of total ethylene = 0.63 Kg/hr
Solvent = 1.45 % of total ethylene = 92.4 Kg/hr
Nitrogen required for drying = 33194 Kg/hr
Hydrogen required (0.099 % ) = 6.31 Kg/hr
Overall Material Balance:
Ethylene gas
n-hexnae
Catalyst
Hydrogen
Ethylene gas
n-hexnae
Catalyst
Hydrogen
HDPE powder
Plant
Components Entering, Kg/hr Leaving, Kg/hr
Ethylene gas 6375 127.5
n-hexane, solvent 92.4 92.4
Catalyst 0.631 0.631
Hydrogen gas 6.31 6.31
HDPE ---- 6253.5
Nitrogen 33194 33194
Total 39668.341 39668.341
Mixer
Component Entering Kg/hr Leaving Kg/hr
Catalyst 0.631 0.631
n-hexane 94.4 92.4
Total 93.031 93.031
1
st
REACTOR:
Component
(7)
Entering, Kg/hr Leaving, Kg/hr
Ethylene gas 6375 382.5
Catalyst 0.631 0.031
n-hexane, solvent 92.4 92.4
Un complete HDPE ----- 5993.1
Total 6468.03 6468.03
2
nd
REACTOR:
Component Entering, Kg/hr Leaving, Kg/hr
Ethylene from R-1 382.5 127.5
n-hexane from R-1 92.4 92.4
Catalyst from R-1 0.031 0.0001
HDPE from R-1 5993.1 6254.41
Hydrogen gas 6.31 0.31
Total 6474.34 6474.12
CENTRIFUGE
Component Entering, Kg/hr Leaving, Kg/hr
HDPE 6254.41 6274.05
Catalyst 0.0001 0.631
n-hexane 92.4 73.92
Total 6348.61 6348.61
DRYER
Component Entering, Kg Leaving, Kg
HDPE 6253.77 6253.77
n-hexane contents 18.48 18.48
Nitrogen 33194 33194
Total 39466.25 39466.25
You might also like
- Basell PPDocument32 pagesBasell PPpr9842100% (3)
- Polymer Production TechnologyDocument12 pagesPolymer Production TechnologyMohd ImranNo ratings yet
- Additives and CompoundingDocument117 pagesAdditives and CompoundingShubham ChaudharyNo ratings yet
- PolypropyleneDocument5 pagesPolypropylenenitesh_mpsNo ratings yet
- Isocyanate SDocument67 pagesIsocyanate SA Mahmood100% (4)
- Polyethylene Production Technologies PDFDocument81 pagesPolyethylene Production Technologies PDFJelssy Huaringa Yupanqui100% (1)
- Polymerization Reactions ExplainedDocument111 pagesPolymerization Reactions ExplainedHamsiah Sayah100% (1)
- Polypropylene PDFDocument36 pagesPolypropylene PDFabdul qahar100% (1)
- Pipe Flow Hydraulics Webinar PresentationDocument51 pagesPipe Flow Hydraulics Webinar Presentationmukhzinrashid100% (1)
- 19.02.additives For PlasticsDocument61 pages19.02.additives For Plasticsjraman24No ratings yet
- Ethylene Polymers, LLDPEDocument42 pagesEthylene Polymers, LLDPEflsaucedo100% (2)
- Lummus PolypropyleneDocument2 pagesLummus PolypropyleneÜmit Düngel100% (1)
- Styrene MonomerDocument13 pagesStyrene MonomerSerkan Gecim100% (1)
- PVC Technology: Chapter 4Document37 pagesPVC Technology: Chapter 4AndriNo ratings yet
- Production of High Quality FoamDocument73 pagesProduction of High Quality FoamMOHAMMED YUSUF MAIAGOGONo ratings yet
- PolyethyleneDocument38 pagesPolyethyleneGuery Saenz80% (5)
- Lecture 2production of PolyolefinsDocument12 pagesLecture 2production of Polyolefinsrk_gummaluri5334No ratings yet
- HDPE-LLDPE Notes For MTechDocument4 pagesHDPE-LLDPE Notes For MTechSdkmega HhNo ratings yet
- Unipol Polypropylene Process Garners LicenseesDocument3 pagesUnipol Polypropylene Process Garners LicenseesridanormaNo ratings yet
- Polyethylene Production Technologies-LibreDocument81 pagesPolyethylene Production Technologies-LibreSchwanSty100% (2)
- Handbook of PolypropyleneDocument573 pagesHandbook of Polypropylenemarktanner100% (7)
- Worksheet Gases III Answers 1Document5 pagesWorksheet Gases III Answers 1Emilio JacintoNo ratings yet
- Full Report PolypropyleneDocument9 pagesFull Report PolypropyleneAin FarhanaNo ratings yet
- Seminar On "Flame Retardent Synthetic Fibres": By: Raghav Mehra Mtech 1 YearDocument49 pagesSeminar On "Flame Retardent Synthetic Fibres": By: Raghav Mehra Mtech 1 YearRaghav MehraNo ratings yet
- Manufacturing of Linear Low Density Polyethylene (LldpeDocument8 pagesManufacturing of Linear Low Density Polyethylene (LldpeMarut DuttNo ratings yet
- Unipol PPDocument12 pagesUnipol PPyamakun100% (2)
- Polyvinylchloride — 2: Main Lectures Presented at the Second International Symposium on Polyvinylchloride, Lyon-Villeurbanne, France, 5 - 9 July 1976From EverandPolyvinylchloride — 2: Main Lectures Presented at the Second International Symposium on Polyvinylchloride, Lyon-Villeurbanne, France, 5 - 9 July 1976A. GuyotNo ratings yet
- PolypropyleneDocument9 pagesPolypropylenePearl PrakashNo ratings yet
- Polymerization Techniques: Bulk, Solution, Suspension & EmulsionDocument38 pagesPolymerization Techniques: Bulk, Solution, Suspension & EmulsionTej Pratap SinghNo ratings yet
- Reactive ExtrusionDocument23 pagesReactive ExtrusionDIPAK VINAYAK SHIRBHATENo ratings yet
- PolyethyleneDocument17 pagesPolyethylenePrateek Mall100% (1)
- PolypropyleneDocument3 pagesPolypropyleneRohit WadhwaniNo ratings yet
- Introduction To PolyethyleneDocument19 pagesIntroduction To PolyethyleneChiu FongNo ratings yet
- Polyurethane Foam ApplicationDocument5 pagesPolyurethane Foam ApplicationMichelle EvelynNo ratings yet
- PolypropyleneDocument29 pagesPolypropyleneZeny Naranjo100% (1)
- Additives for Polyolefins: Getting the Most out of Polypropylene, Polyethylene and TPOFrom EverandAdditives for Polyolefins: Getting the Most out of Polypropylene, Polyethylene and TPONo ratings yet
- Polyethylene: Shriguru-17BEC0803 Kumar Shantanu-17BEC0208 Mrintunjay Pathak-17BEC0054Document22 pagesPolyethylene: Shriguru-17BEC0803 Kumar Shantanu-17BEC0208 Mrintunjay Pathak-17BEC0054ShriGuru B HugarNo ratings yet
- Design and Study of Manufacturing of Polyester Plant Using Pta and Meg-Ijaerdv04i0425431 PDFDocument8 pagesDesign and Study of Manufacturing of Polyester Plant Using Pta and Meg-Ijaerdv04i0425431 PDFMary Grace Velitario100% (1)
- Characteristics of PETDocument3 pagesCharacteristics of PETasyechleniNo ratings yet
- Production of PolyethyleneDocument57 pagesProduction of PolyethyleneFarid IskandarNo ratings yet
- Technology Economics Polypropylene Via Gas Phase ProcessDocument78 pagesTechnology Economics Polypropylene Via Gas Phase ProcessTato Flores0% (5)
- Review On Development of Polypropylene Manufacturing ProcessDocument11 pagesReview On Development of Polypropylene Manufacturing ProcessShweta Yadav100% (1)
- Lyondell Tubular Vs Autoclave 1 PDFDocument2 pagesLyondell Tubular Vs Autoclave 1 PDFJacky V. HerbasNo ratings yet
- BTPDocument15 pagesBTPPARTH NAGARNo ratings yet
- Polyethylene - Chemistry and Production ProcessesDocument26 pagesPolyethylene - Chemistry and Production Processeschiuchan888No ratings yet
- Polymer EngineeringDocument33 pagesPolymer EngineeringDiana Isis Velasco100% (1)
- Linear Low Density PolyethyleneDocument12 pagesLinear Low Density PolyethylenedaempiNo ratings yet
- All of PPDocument6 pagesAll of PPUmadNo ratings yet
- Compounding Additives: in This ChapterDocument17 pagesCompounding Additives: in This Chaptermonalihania100% (1)
- Polyethylene Properties - VinidexDocument8 pagesPolyethylene Properties - VinidexalexNo ratings yet
- Ethylene GlycolDocument4 pagesEthylene GlycolAli AhsanNo ratings yet
- Additives For Plastics Handbook - (Chapter 3. The World Market)Document6 pagesAdditives For Plastics Handbook - (Chapter 3. The World Market)Pablo Fernández SaavedraNo ratings yet
- POLY-OLEFINS by LOW PRESSURE Ziegler Process: Aluminum Alkyl Drier Light Ends DiluentDocument3 pagesPOLY-OLEFINS by LOW PRESSURE Ziegler Process: Aluminum Alkyl Drier Light Ends Diluentessakkiraj.mNo ratings yet
- Polymers and Plastics Technology HandbookDocument5 pagesPolymers and Plastics Technology HandbookAwais GeeNo ratings yet
- Classification of PolymerDocument23 pagesClassification of PolymerChaudhary Asheesh RahalNo ratings yet
- PVCDocument18 pagesPVCCemal KayaNo ratings yet
- Metallocene Linear Low-Density Polyethylene Properties and ApplicationsDocument15 pagesMetallocene Linear Low-Density Polyethylene Properties and ApplicationsRodriguez JohannNo ratings yet
- Plastic AdditivesDocument824 pagesPlastic AdditivesThai Thi Hong Loan75% (4)
- Material Science Chapter on Polymer Types, Processing & ApplicationsDocument13 pagesMaterial Science Chapter on Polymer Types, Processing & ApplicationsVaibhav ShrivastavaNo ratings yet
- PolyethyleneDocument11 pagesPolyethyleneIvan German Ramos100% (1)
- How to Name an Inorganic Substance: A Guide to the Use of Nomenclature of Inorganic Chemistry: Definitive Rules 1970From EverandHow to Name an Inorganic Substance: A Guide to the Use of Nomenclature of Inorganic Chemistry: Definitive Rules 1970Rating: 5 out of 5 stars5/5 (1)
- Energy Conversion and Management: Mohamed Lachheb, Mustapha Karkri, Fethi Albouchi, Foued Mzali, Sassi Ben NasrallahDocument9 pagesEnergy Conversion and Management: Mohamed Lachheb, Mustapha Karkri, Fethi Albouchi, Foued Mzali, Sassi Ben NasrallahsaltyNo ratings yet
- Adhesives and Sealants 12 - 2018Document6 pagesAdhesives and Sealants 12 - 2018Jose LopezNo ratings yet
- 1 - Basic Concepts For Simple and Complex FluidsDocument3 pages1 - Basic Concepts For Simple and Complex FluidszhangNo ratings yet
- Wave OpticsDocument33 pagesWave OpticsmisspayujiNo ratings yet
- Vibrationally-resolved electronic spectra in GAUSSIAN 09Document20 pagesVibrationally-resolved electronic spectra in GAUSSIAN 09Axel Velasco ChávezNo ratings yet
- Cambridge International Advanced Subsidiary and Advanced LevelDocument12 pagesCambridge International Advanced Subsidiary and Advanced LevelRahi FurqanNo ratings yet
- Effect of Ultrasonic Irradiation Treatment On Rheological BehaviourDocument17 pagesEffect of Ultrasonic Irradiation Treatment On Rheological BehaviourSOCRATESNo ratings yet
- COA Dark ChocolateDocument3 pagesCOA Dark ChocolatejavinjayaofficeNo ratings yet
- 4140 Inorganic Anions by Capillary Ion Electrophoresis (Editorial Revisions, 2011)Document9 pages4140 Inorganic Anions by Capillary Ion Electrophoresis (Editorial Revisions, 2011)TaniaCarpioNo ratings yet
- GRADE 8 CHEMISTRY Explaining PressureDocument15 pagesGRADE 8 CHEMISTRY Explaining PressuredodoNo ratings yet
- Liquid Cooling Ebook2Document16 pagesLiquid Cooling Ebook2Ashish ShuklaNo ratings yet
- Energy Saving Research of Natural Gas Liquefaction Plant Based On Waste Heat Utilization of Gas Turbine ExhaustDocument11 pagesEnergy Saving Research of Natural Gas Liquefaction Plant Based On Waste Heat Utilization of Gas Turbine ExhaustAbelardo Nardo Guzmán LavadoNo ratings yet
- Productcatalog YAH en PUBL-5302 (0112)Document14 pagesProductcatalog YAH en PUBL-5302 (0112)Bryan ChiaNo ratings yet
- Adsorption IsothermsDocument3 pagesAdsorption IsothermsKrushit PatelNo ratings yet
- Bonding (p1)Document22 pagesBonding (p1)HashimNo ratings yet
- Hidróxido de Sodio: Nombre: Ruber Torrez TupaDocument2 pagesHidróxido de Sodio: Nombre: Ruber Torrez TupaJessica FloresNo ratings yet
- Materials Chemistry C: Journal ofDocument7 pagesMaterials Chemistry C: Journal ofMohon MaapNo ratings yet
- Design and Performance Evaluation of An Ice Block Making MachineDocument9 pagesDesign and Performance Evaluation of An Ice Block Making Machinebasel abduNo ratings yet
- Dost Science Reviewer IIDocument6 pagesDost Science Reviewer IIEster Joy BordajeNo ratings yet
- Introduction to Chemical Engineering Thermodynamics IDocument58 pagesIntroduction to Chemical Engineering Thermodynamics IGlory UsoroNo ratings yet
- Assignment 2Document1 pageAssignment 2Ankit OlaNo ratings yet
- Bahadur 2003Document10 pagesBahadur 2003Thanh Uyen LeNo ratings yet
- Electron Configurations Practice-17Document3 pagesElectron Configurations Practice-17api-368121935No ratings yet
- Test Bank For Chemistry A Molecular Approach 4th Edition by Tro ISBN 0134112830 9780134112831Document36 pagesTest Bank For Chemistry A Molecular Approach 4th Edition by Tro ISBN 0134112830 9780134112831shawnramirez06042001jzc100% (23)
- Polybutadiene Rubber Properties and UsesDocument3 pagesPolybutadiene Rubber Properties and UsesHamzah A. LaftaNo ratings yet
- The Law of Conservation of EnergyDocument3 pagesThe Law of Conservation of EnergyChahatBhattiAliNo ratings yet
- Characteristic Reactions of Organic HalidesDocument6 pagesCharacteristic Reactions of Organic HalidesJules Patrick JacobNo ratings yet
- AMPLOP PEskam-dikonversiDocument29 pagesAMPLOP PEskam-dikonversiahlan habibiNo ratings yet