Professional Documents
Culture Documents
تصحيح: امتحان تجريبي للباكلوريا الجزائرية * شعبة العلوم التجريبية: النموذج 3
Uploaded by
الغزيزال الحسن EL GHZIZAL HassaneOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
تصحيح: امتحان تجريبي للباكلوريا الجزائرية * شعبة العلوم التجريبية: النموذج 3
Uploaded by
الغزيزال الحسن EL GHZIZAL HassaneCopyright:
Available Formats
اﻟﻌﻼﻣﺔ ﺣﻠﻮل اﻟﺘﻤﺎرﯾﻦ
ﺍﻟﺘﻤﺭﻴﻥ ﺍﻷﻭل ) 2.5ﻨﻘﻁﺔ(
HCOOH / HCOO - ; CH 3COOH / CH 3COO - .1ﺍﻟﺜﻨﺎﺌﻴﺘﺎﻥ ﺍﻟﻤﺸﺎﺭﻜﺘﺎﻥ ﻓﻲ ﺍﻟﺘﻔﺎﻋل ﻫﻤﺎ:
اﻟﻤﻌﺎدﻟﺘﺎن اﻟﻨﺼﻔﯿﺘﺎن اﻟﻤﻮاﻓﻘﺘﺎن ﻟﮭﻤﺎ:
¼ ) CH 3COOH ( aq ) = CH 3COO - ( aq ) + H + (aq
¼ ) HCOOH ( aq ) = HCOO - ( aq ) + H + (aq
.2ﻨﺤﺼل ﻋﻠﻰ ﻤﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻋل ﺒﻴﻥ ﺤﻤﺽ ﺍﻟﻤﻴﺜﺎﻨﻭﻴﻙ ﺸﻭﺍﺭﺩ ﺍﻹﻴﺜﺎﻨﻭﺍﺕ ﺍﻨﻁﻼﻗﺎ ﻤﻥ
ﺍﻟﻤﻌﺎﺩﻟﺘﻴﻥ ﺍﻟﻨﺼﻔﻴﺘﻴﻥ ﺍﻟﺒﺭﻭﺘﻭﻨﻴﺘﻴﻥ:
½ ) CH 3 COO (aq ) + HCOOH (aq ) = HCOO - (aq ) + CH 3COOH (aq
-
.3ﺜﺎﺒﺕ ﺍﻟﺘﻭﺍﺯﻥ :
=K
(
Ka1 HCOOH / HCOO -
) =
10- pka1
¼ (
Ka2 CH 3COOH / CH 3COO - ) 10- pka2
10-3,8
=K = 7,9
¼ 10-4,7
.4ﻜﺴﺭ ﺍﻟﺘﻔﺎﻋل ﻓﻲ ﺍﻟﺤﺎﻟﺔ ﺍﻻﺒﺘﺩﺍﺌﻴﺔ:
Qr ,i =
[HCOO ]i × [CH 3 COOH ]i
-
¼ [CH 3COO - ]i × [HCOOH ]i
æ 2,0 ´ 10 - 2 ö æ 2,0 ´ 10 - 2 ö
çç ÷÷ × çç ÷÷
¼ Qr ,i =
è V ø è V ø =1
æ 2,0 ´ 10 - 2 ö æ 2,0 ´ 10 - 2 ö
çç ÷÷ × çç ÷÷
è V ø è V ø
¼ . ﻗﻴﻤﺔ K : Qr ,i < K .5ﻴﻨﺘﻬﻲ ﻜﺴﺭ ﺍﻟﺘﻔﺎﻋل ﻨﺤﻭ ﺜﺎﺒﺕ ﺍﻟﺘﻭﺍﺯﻥ ﻭ ﻴﺯﺩﺍﺩ ﺇﻟﻰ ﺃﻥ ﻴﺒﻠﻎ
¼ ﺘﺘﻁﻭﺭ ﺍﻟﺠﻤﻠﺔ ﻓﻲ ﺍﻻﺘﺠﺎﻩ ﺍﻟﻤﺒﺎﺸﺭ ،ﺇﺫﻥ ﻴﺘﺸﻜل ﺤﻤﺽ ﺍﻹﻴﺜﺎﻨﻭﻴﻙ.
ﺍﻟﺘﻤﺭﻴﻥ ﺍﻟﺜﺎﻨﻲ3,5 ):ﻨﻘﻁﺔ(
.1ﺃ .ﻴﻌﻁﻲ ﺘﻁﺒﻴﻕ ﻗﺎﻨﻭﻥ ﺠﻤﻊ ﺍﻟﺘﻭﺘﺭﺍﺕ ﻓﻲ ﺩﺍﺭﺓ ﺍﻟﻤﻭﻟﺩ:
¼ u PA + u AB + u BM + u MP = 0
¼ 0 + u AB + R × i - E = 0 Þ u AB + R × i = E
dq du
ﻟﻜﻥi = A = C × AB :
dt dt
du
ﻭ ﻤﻨﻪu AB + RC × AB = E :
¼ dt
ﻭ ﺒﻭﻀﻊt = RC :
du
¼ ﻴﺄﺘﻲu AB + t × AB = E :
dt
PDF created with pdfFactory Pro trial version www.pdffactory.com
¼ ﺏ .ﺘﺒﻴﻥ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻀﻠﻴﺔ ﺍﻷﺨﻴﺭﺓ ﺃﻥ t = RCﻴﻘﺩﺭ ﺒﺎﻟﺜﺎﻨﻴﺔ ،ﺃﻥ ﺤﺩﻱ ﺍﻟﻁﺭﻑ ﺍﻷﻭل ﻤﻥ
ﺍﻟﻤﻌﺎﺩﻟﺔ ﻴﺠﺏ ﺃﻥ ﻴﻜﻭﻨﺎ ﻤﻘﺩﺭﻴﻥ ﺒﺎﻟﻔﻭﻟﻁ ﻜﺎﻟﻁﺭﻑ ﺍﻟﺜﺎﻨﻲ ﻤﻥ ﺍﻟﻤﻌﺎﺩﻟﺔ.
ﻴﺴﻤﺢ ﺍﻟﺘﺤﻠﻴل ﺍﻟﺒﻌﺩﻱ ﺒﺎﻟﻭﺼﻭل ﺇﻟﻰ ﻫﺫﻩ ﺍﻟﻨﺘﻴﺠﺔ:
-ﻤﻥ ﻗﺎﻨﻭﻥ ﺃﻭﻡ ، U = R × Iﻨﺠﺩ ﺃﻥ. [R ] = [U ]× [I ]-1 :
-ﻭ ﻤﻥ ﺍﻟﻌﻼﻗﺔ ، i = C × duﻨﺠﺩ ﺃﻥ. [C ] = [I ] × [T ]× [U ]-1 :
dt
] [RC ] = [R] × [C ] = [T
ﻨﺴﺘﻨﺘﺞ ﺇﺫﻥ ﺃﻥ:
¼ ﻓﺎﻟﺠﺩﺍﺀ t = RCﻟﻪ ﺒﻌﺩ ﺍﻟﺯﻤﻥ ،ﻭ ﺒﺎﻟﺘﺎﻟﻲ ﻓﻬﻭ ﻴﻘﺩﺭ ﺒﺎﻟﺜﺎﻨﻴﺔ.
t
-
) u AB = E (1 - e t .2
t t
du AB E - E -
= 0+ ×e t
= ×e t
ﺇﺫﻥ:
¼ dt t t
ﺒﺎﻟﺘﻌﻭﻴﺽ ﻓﻲ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻀﻠﻴﺔ ،ﻨﺠﺩ:
æ E -t ö -
t
t × çç × e t ÷ + E - E ×e t = E
÷
èt ø
t
-
¼ ﺇﺫﻥ u AB = E (1 - e t ) :ﻴﺤﻘﻕ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻀﻠﻴﺔ ،ﻓﻬﻭ ﺤل ﻟﻬﺎ.
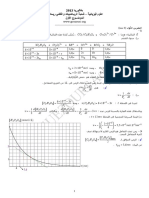
U A B (V ) .3ﺃ .ﺸﻜل ﺍﻟﻤﻨﺤﻨﻰ ﺍﻟﺒﻴﺎﻨﻲ
ﺇﺤﺩﺍﺜﻴﺎ ﻨﻘﻁﺔ ﺘﻘﺎﻁﻊ ﺍﻟﻤﻤﺎﺱ
H ﻟﻠﻤﻨﺤﻨﻰ ﻋﻨﺩ ﺍﻟﻤﺒﺩﺃ ﻤﻊ ﺍﻟﺨﻁ ﺍﻟﻤﻘﺎﺭﺏ
E
¼ ﻫﻤﺎ:
uH = E
tH = t = R × C
¼ ﺇﺫﻥ :
u H = 100V
t H = 5,0 ´ 10 -3 s
¼ t = R ×C ) t (s -
t
.5ﻤﻥ
ﺍﻟﻌﻼﻗﺔ ) u AB = E (1 - e t
-ﻟﻤﺎ u AB = 0 : t = 0
-ﻟﻤﺎ u AB = 0,63E = 63V : t1 = t
-ﻟﻤﺎ u AB = 0,993E = 99,3V : t 2 = 5t
½
-ﻟﻤﺎ u AB : t ® ¥ﺘﻨﺘﻬﻲ ﻨﺤﻭ . E = 100V
ﻨﺴﺘﻨﺘﺞ ﺃﻨﻪ ﺨﻼل ﺯﻤﻥ ﻴﺴﺎﻭﻱ ﺍﻟﺜﺎﺒﺕ t = RCﻓﺈﻥ ﺸﺤﻨﺔ ﺍﻟﻤﻜﺜﻔﺔ ﺘﺒﻠﻎ 63%ﻤﻥ ﻗﻴﻨﺘﻬﺎ
ﺍﻟﺤﺩﻴﺔ ﻭ ﺃﻨﻪ ﺨﻼل ﺯﻤﻥ ، t = 5tﻓﺈﻥ ﺸﺤﻨﺘﻬﺎ ﺘﺘﺠﺎﻭﺯ 99%ﻤﻥ ﻗﻴﻤﺘﻬﺎ ﺍﻟﺤﺩﻴﺔ.
¼
ﺍﻟﺘﻤﺭﻴﻥ ﺍﻟﺜﺎﻟﺙ ) 4ﻨﻘﺎﻁ( :
b æ t
ö
) x(t ) = × (1 - e -at
-
ﻤﻊ ﺍﻟﻤﻌﺎﺩﻟﺔ v(t ) = 1,14 × 1 - e
ç ÷ 0 ,132
.1ﺃ .ﺒﻤﻁﺎﺒﻘﺔ ﺍﻟﻤﻌﺎﺩﻟﺔ
a ç ÷
è ø
1 b
½ = .a ﻴﻨﺘﺞ= 1,14 :
0,132 a
PDF created with pdfFactory Pro trial version www.pdffactory.com
b æ -
t
ö
ﻤﺘﺠﺎﻨﺴﺔ ﻤﻊ ﺍﻟﺴﺭﻋﺔ ﻓﻲ ﻋﺒﺎﺭﺓ ﺍﻟﺘﺴﺎﺭﻉ ﻟﻴﺱ ﻟﻪ ﺒﻌﺩﺍ ،ﺇﺫﻥ ﺍﻟﻨﺴﺒﺔ ÷ ç1 - e 0,132 ﺍﻟﺤﺩ
a ç ÷
è ø
¼ . m.s -1 ﻭ ﺒﺎﻟﺘﺎﻟﻲ ﺘﻘﺩﺭ ﺒﻭﺤﺩﺓ ﺍﻟﺴﺭﻋﺔ ﺃﻱ
æ -
t
ö
ﻫﻲ ﺤل ﻟﻤﻌﺎﺩﻟﺔ ﺘﻔﺎﻀﻠﻴﺔ ﻤﻥ ﺍﻟﻨﻭﻉ: v(t ) = 1,14 × 1 - e
ç ÷ 0 ,132
ﺏ .ﺍﻟﻤﻌﺎﺩﻟﺔ
ç ÷
è ø
¼ dx
+a × x = b
dt
dv
ﺒﺎﻟﻤﻁﺎﺒﻘﺔ ، x Û vﺃﻱ+ a × v = b :
¼ dt
b
ﺃﻱ b = 1,14a ﻟﻜﻥ= 1,14 a = 7,58 :
a
¼ ﺇﺫﻥb = 1,14 ´ 7,58 = 8,64 :
dv
¼ . ﺍﻟﻤﻌﻁﺎﺓ + 7,58v = 8,64 ﺒﻘﻴﻤﺘﻬﺎ ﻨﺼل ﺇﻟﻰ ﺍﻟﻌﺒﺎﺭﺓ ﺒﺘﻌﻭﻴﺽ b a
dt
.2ﺃ .ﺍﻟﺠﻤﻠﺔ ﺍﻟﻤﺩﺭﻭﺴﺔ ﻫﻲ ﺍﻟﻜﺭﺓ ﻓﻲ ﺍﻟﻤﺭﺠﻊ ﺍﻷﺭﻀﻲ ﺍﻟﺫﻱ ﻨﻔﺘﺭﻀﻪ ﻏﺎﻟﻴﻠﻴﺎ.
r ﺍﻟﻘﻭﻯ ﺍﻟﻤﻁﺒﻘﺔ ﻋﻠﻰ ﺍﻟﻜﺭﺓ ﻫﻲ:
¼ f
r -ﺍﻟﺜﻘل ، P = m × gﻤﻨﺤﺎﻫﺎ ﺸﺎﻗﻭﻟﻲ ﻭ ﺍﺘﺠﺎﻫﻬﺎ ﻨﺤﻭ ﺍﻷﺴﻔل.
p
-ﺩﺍﻓﻌﺔ ﺃﺭﺨﻤﻴﺩﺱ ، pﻤﻨﺤﺎﻫﺎ ﺸﺎﻗﻭﻟﻲ ﻭ ﺍﺘﺠﺎﻫﻬﺎ ﻨﺤﻭ ﺍﻷﻋﻠﻰ.
¼ -ﻗﻭﻯ ﺍﻹﺤﺘﻜﺎﻙ ، fﻤﻨﺤﺎﻫﺎ ﺸﺎﻗﻭﻟﻲ ﻭ ﺍﺘﺠﺎﻫﻬﺎ ﻨﺤﻭ ﺍﻷﻋﻠﻰ.
r
P
¼ åF ext ﺏ .ﺒﺘﻁﺒﻴﻕ ﻗﺎﻨﻭﻥ ﻨﻴﻭﺘﻥ ﺍﻟﺜﺎﻨﻲ:
= P + f + p = m × aG
¼ . P - f -p = m×a ﺒﺎﻹﺴﻘﺎﻁ ﻋﻠﻰ ﺍﻟﻤﺤﻭﺭ ﺍﻟﺸﺎﻗﻭﻟﻲ ﺍﻟﻤﻭﺠﻪ ﻨﺤﻭ ﺍﻷﺴﻔل:
.3ﺃ .ﺒﺎﻟﺘﻌﻭﻴﺽ ﻋﻥ fﻭ pﻓﻲ ﺍﻟﻌﺒﺎﺭﺓ ﺍﻷﺨﻴﺭﺓ ،ﻨﺠﺩ:
dv
× m × g - k × v - r ×V × g = m
dt
dv
= g × ( m - r ×V ) - k × v
¼ dt
ﻴﻨﺘﺞ: ﻋﻠﻰ m ﻭ ﺒﻘﺴﻤﺔ ﻁﺭﻓﻲ ﺍﻟﻤﻌﺎﺩﻟﺔ
¼ dv k æ r ×V ö
+ × v = ç1 - ÷ .g
dt m è m ø
ﺏ .ﺒﻤﻁﺎﺒﻘﺔ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﺴﺎﺒﻘﺔ ﻤﻊ ﺍﻟﻤﻌﺎﺩﻟﺔ ، dx + a × x = bﻨﺠﺩ:
dt
¼ k æ r ×V ö
=a ﻭ b = ç1 - ÷ .g
m è m ø
¼ r ×V = 0 ،ﻓﺈﻥ: ﻤﻌﺩﻭﻤﺔ p = 0 ﺝ .ﺇﺫﺍ ﻜﺎﻨﺕ ﺩﺍﻓﻌﺔ ﺃﺭﺨﻤﻴﺩﺱ
b = 9,80 m.s -2 ﻭﻤﻨﻪ: b=g ﻭ ﻤﻨﻪ ، b = æç1 - 0 ö÷.g :ﺃﻱ ﺃﻥ:
è mø
dv
¼ b ¹ g ¹ 9,80m.s ² ﺇﺫﻥ: b = 8, 64 m.s -2 ﺃﻥ + 7,58 v = 8, 64 ﻨﻼﺤﻅ ﻓﻲ ﺍﻟﻤﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻀﻠﻴﺔ:
dt
PDF created with pdfFactory Pro trial version www.pdffactory.com
ﻭ ﻋﻠﻴﻪ ﻓﺈﻥ ﻴﺠﺏ ﺃﺨﺫ ﺩﺍﻓﻌﺔ ﺃﺭﺨﻤﻴﺩﺱ ﻓﻲ ﺍﻟﺤﺴﺒﺎﻥ ﺤﻴﺙ ﺘﺒﻠﻎ ﺸﺩﺘﻬﺎ:
p = m × (g - b ) = 3,7 ´ 10 -2 N
ﺍﻟﺘﻤﺭﻴﻥ ﺍﻟﺭﺍﺒﻊ ) 2ﻧﻘﻄﺔ( :
½ -1ﻴﺭﺠﻊ ﺘﻨﺎﻗﺹ ﺍﻟﺴﻌﺔ ﺇﻟﻰ ﺍﻟﻤﻘﺎﻭﻤﺔ ﺍﻟﺩﺍﺨﻠﻴﺔ ﻟﻠﻭﺸﻴﻌﺔ ،ﺤﻴﺙ ﺘﺘﺤﻭل ﺍﻟﻁﺎﻗﺔ ﻋﻠﻰ ﺸﻜل
ﺤﺭﺍﺭﺓ ﺒﻔﻌل ﺠﻭل.
-2ﻨﻼﺤﻅ ﻤﻥ ﺍﻟﺒﻴﺎﻥ ﺃﻥ5T = 100 ms :
¼ ﻭ ﻤﻨﻪ :ﻗﻴﻤﺔ ﺸﺒﻪ ﺍﻟﺩﻭﺭ. T = 20 ms :
-3ﺍﻟﺩﻭﺭ ﺍﻟﺫﺍﺘﻲ ﻟﻪ ﻨﻔﺱ ﻗﻴﻤﺔ ﺸﺒﻪ ﺍﻟﺩﻭﺭ ،ﺇﺫﻥT = T0 = 2p × L × C :
½ ﺃﻱ ﺃﻥT ² = 4p ² × L × C :
T²
=C ﻭ ﻤﻨﻪ:
4p ² × L
½
=C
( 20 ´10 ) ² = 10 ´10
-3
-6
F = 10m F
4 ´ 10 ´1, 0
¼ ﻫﺫﻩ ﺍﻟﻘﻴﻤﺔ ﻤﺘﻁﺎﺒﻘﺔ ﻤﻊ ﺍﻟﻘﻴﻤﺔ ﺍﻟﺘﻲ ﺃﻋﻁﻬﺎ ﺍﻟﺼﺎﻨﻊ.
)ﺍﻟﺘﻤﺭﻴﻥ ﺍﻟﺨﺎﻤﺱ 04).ﻨﻘﺎﻁ(:
¼ -1ﺃ /ﺍﻟﺴﻠﻙ ﺫﻭ ﺍﻟﻘﻁﺭ 2,0mmﻴﻭﻗﻑ ﺤﺯﻤﺔ ﺍﻟﻠﻴﺯﺭ.
ﺏ /ﻋﻨﺩ ﺍﺴﺘﻌﻤﺎل ﺴﻠﻙ ﻗﻁﺭﻩ ، 0,080mmﻨﻼﺤﻅ ﻅﺎﻫﺭﺓ ﺍﻹﻨﻌﺭﺍﺝ ﻤﺸﺎﺒﻬﺔ ﻟﺘﻠﻙ ﺍﻟﺘﻲ
ﺘﺤﺼل ﺒﺎﺴﺘﻌﻤﺎل ﺸﻕ ﻋﺭﻀﻪ . a
½ ﺘﺘﺸﻜل ﻋﺩﺓ ﺒﻘﻊ ﻀﻭﺌﻴﺔ ﻤﻭﺯﻋﺔ ﻋﻠﻰ ﻤﺤﻭﺭ ﻋﻤﻭﺩﻱ ﻋﻠﻰ ﻤﻨﺤﻨﻰ ﺍﻟﺴﻠﻙ ﻭ ﺘﻜﻭﻥ
ﺍﻟﺒﻘﻌﺔ ﺍﻟﻤﺭﻜﺯﻴﺔ ﺃﺸﺩ ﺇﻀﺎﺀﺓ ﻭ ﺘﺘﻨﺎﻗﺹ ﺸﺩﺓ ﺇﻀﺎﺀﺓ ﺍﻟﺒﻘﻊ ﺍﻷﺨﺭﻯ ﻋﻠﻰ ﺤﺎﻓﺘﻲ ﺍﻟﺸﺎﺸﺔ.
q
l
½
D
l
½ =q ﺃ/ﺍﻟﻌﻼﻗﺔ ﺍﻟﺘﻲ ﺘﺭﺒﻁ ﺒﻴﻥ a l qﻫﻲ: -2
a
ﺏ /ﻤﻥ ﺃﺠل ﺯﺍﻭﻴﺔ qﺼﻐﻴﺭﺓ: tan q » q (rad ) ،
¼ =q
l
2D
2D × l
½ =l ﺇﺫﻥ:
a
PDF created with pdfFactory Pro trial version www.pdffactory.com
¼ a ×l
=l -3ﺃ /ﻤﻥ ﺍﻟﻌﻼﻗﺔ ﺍﻟﺴﺎﺒﻘﺔ ،ﻨﺠﺩ:
2D
0,080 ´ 10 -3 ´ 6,5 ´ 10 -2
=l = 6,34 ´ 10 -7 m ﻴﻌﻁﻲ ﺍﻟﺘﻁﺒﻴﻕ ﺍﻟﻌﺩﺩﻱ:
2 ´ 4,10
l = 6,34 ´ 10 -7 m = 0,634 mm
¼
ﺏ /ﻴﻜﻭﻥ ﻁﻭل ﺍﻟﻤﻭﺠﺔ lﻤﺤﺼﻭﺭﺍ ﻓﻲ ﺍﻟﻤﺠﺎل ﺍﻟﺘﺎﻟﻲ:
0,079 ´ 10 -3 ´ 6, 4 ´ 10 -2 0,081 ´ 10 -3 ´ 6,6 ´ 10 -2
<<l
2 ´ 4,15 2 ´ 4,05
½ 0,61mm < l < 0,66mm ﺃﻱ:
¼ ﺇﺫﺍ ﺃﺨﺫﻨﺎ ﻓﻲ ﺍﻟﺤﺴﺒﺎﻥ ﺍﻹﺭﺘﻴﺎﺒﺎﺕ ﻓﻲ ﺍﻟﻘﻴﺎﺱ ،ﻓﺈﻥ ﻫﺫﻩ ﺍﻟﻘﻴﻤﺔ ﺘﻜﻭﻥ ﻋﻠﻰ ﺘﻭﺍﻓﻕ ﻤﻊ ﺍﻟﻘﻴﻤﺔ
ﺍﻟﺘﻲ ﺤﺩﺩﻫﺎ ﺍﻟﺼﺎﻨﻊ ) . (0,633mm
¼ . 0,02
´ 100 = 3% ﺩﻗﺔ ﺍﻟﻘﻴﺎﺱ ﻫﻲ:
0,634
ﺍﻟﺘﻤﺭﻴﻥ ﺍﻟﺴﺎﺩﺱ04).ﻧﻘﺎط(:
/1-Iﻣﻌﺎدﻟﺔ ﺗﻔﻜﻚ اﻟﻤﺎء اﻷﻛﺴﺠﯿﻨﻲ:
¼ ) 2 H 2 O2 (aq ) ® 2 H 2 O(l ) + O2 ( g
/2ﻜﻤﻴﺔ ﻤﺎﺩﺓ ﺜﻨﺎﺌﻲ ﺍﻷﻜﺴﺠﻴﻥ ﺍﻟﻤﻨﻁﻠﻕ:
VO2 10
¼ = ) n(O2 = = 0.45mol
Vm 22,4
/3ﺠﺩﻭل ﺍﻟﺘﻘﺩﻡ:
ﺤﺎﻟﺔ ﺍﻟﺠﻤﻠﺔ ﺍﻟﺘﻘﺩﻡ )2 H 2O2 (aq ® ) 2 H 2O (l + ) O2 ( g
ﺍﻟﺤﺎﻟﺔ ﺍﻻﺒﺘﺩﺍﺌﻴﺔ 0 n 0 0
¼
ﺍﻟﺤﺎﻟﺔ ﺍﻻﻨﺘﻘﺎﻟﻴﺔ x n - 2x 2x x
ﺍﻟﺤﺎﻟﺔ ﺍﻟﻨﻬﺎﺌﻴﺔ ) xmax = n (O2 ) n - 2n ( O2 ) 2n(O2 ) n(O2
ﻜﻤﻴﺔ ﻤﺎﺩﺓ اﻟﻤﺎء اﻷﻛﺴﺠﯿﻨﻲ اﻟﺘﻲ ﺗﺴﻤﺢ ﺑﺎﻧﻄﻼق ﻜﻤﻴﺔ ﻤﺎﺩﺓ ﺜﻨﺎﺌﻲ ﺍﻷﻜﺴﺠﻴﻥ ﻫﻲ:
¼ ) n( H O ) = 2n ( O2
2 2
n( H 2O2 ) = 2 ´ 0, 45 = 0, 9 mol
/4ﺘﻡ ﺤﺴﺎﺏ ﻜﻤﻴﺔ ﺍﻟﻤﺎﺩﺓ اﻧﻄﻼﻗﺎ ﻣﻦ 1 Lﻣﻦ اﻟﻤﺎء اﻷﻛﺴﺠﯿﻨﻲ ،إذن:
PDF created with pdfFactory Pro trial version www.pdffactory.com
¼ ) n ( H 2O2 0,9
=C = = 0,9 mol.L-1
V 1
-IIﺃ/
¼ (
2 ´ MnO4- + 8H + + 5e - = 2Mn 2 + + 4 H 2 O )
¼ (
5 ´ H 2 O2 = O2 + 2 H + + 2e - )
¼ 2MnO4- + 5 H 2 O2 + 6 H + ® 2Mn 2 + + 5O2 + 8 H 2 O
ﺏ/
ﻟﺩﻴﻨﺎ ﻋﻨﺩ ﺒﻠﻭﻍ ﻨﻘﻁﺔ ﺍﻟﺘﻜﺎﻓﺅ :
½ 5 ´ C 0 ´ V0 = 2 ´ C R ´ VR
ﺃﻱ ﺃﻥ:
5 ´ C 0 ´ V0
¼ = CR
2 ´ VR
5 ´ 0,20 ´ 17,9
¼ = CR » 0,9mol.L-1
2 ´ 10,0
ﺠـ/ﻫﺫﻩ ﺍﻟﻘﻴﻤﺔ ﻋﻠﻰ ﺘﻭﺍﻓﻕ ﺘﺎﻡ ﻤﻊ ﺍﻟﻘﻴﻤﺔ ﺍﻟﻤﺤﺴﻭﺒﺔ ﺴﺎﺒﻘﺎ ﻭ ﻗﺩ ﺘﻡ ﺍﺤﺘﺭﺍﻡ ﺍﻟﺩﻻﻟﺔ ﻓﻲ ﺘﺤﻀﻴﺭ
¼ ﻤﺤﻠﻭل اﻟﻤﺎء اﻷﻛﺴﺠﯿﻨﻲ ﻛﻤﺎ ﯾﻨﺒﻐﻲ.
¼ -IIIﺃ /ﺍﻟﺤﺠﻡ ﺍﻟﻤﺴﺘﻌﻤل ﻓﻲ ﻤﻌﺎﻴﺭﺓ ﺍﻟﻤﺤﻠﻭل ﺍﻟﻘﺩﻴﻡ ﺃﺼﻐﺭ ﻤﻤﺎ ﻜﺎﻥ ﻋﻠﻴﻪ ﻓﻲ ﻤﻌﺎﻴﺭﺓ ﺍﻟﻤﺤﻠﻭل
¼ ﻟﻤﺎ ﻜﺎﻥ ﺠﺩﻴﺩﺍ ،ﻫﺫﺍ ﺩﻟﻴل ﻋﻠﻰ ﺃﻥ ﻤﺤﻠﻭل ﺗﻔﻜﻚ اﻟﻤﺎء اﻷﻛﺴﺠﯿﻨﻲ ﺑﻄﻲء.
¼ ب /ﯾﻨﺼﺢ ﺑﺤﻔﻆ اﻟﻘﺎرورة ﻓﻲ ﻣﻜﺎن ﺑﺎرد ﻷن ﺧﻔﺾ درﺟﺔ اﻟﺤﺮارة ﯾﺠﻌﻞ اﻟﺘﻔﺎﻋﻞ أﻛﺜﺮ ﺑﻂء.
PDF created with pdfFactory Pro trial version www.pdffactory.com
You might also like
- الكهرباء: الدارات الكهربائية المحتوية على مكثف وموصل أومي أو وشيعة وموصل أومي: دروس وتمارين مصححةDocument48 pagesالكهرباء: الدارات الكهربائية المحتوية على مكثف وموصل أومي أو وشيعة وموصل أومي: دروس وتمارين مصححةالغزيزال الحسن EL GHZIZAL Hassane86% (7)
- امتحان تجريبي للباكلوريا الجزائرية * شعبة العلوم التجريبية: النموذج 3Document5 pagesامتحان تجريبي للباكلوريا الجزائرية * شعبة العلوم التجريبية: النموذج 3الغزيزال الحسن EL GHZIZAL Hassane100% (35)
- الفيزياء والكيمياء علوم فيزيائية 2020 الدورة الإستدراكية - التصحيحDocument10 pagesالفيزياء والكيمياء علوم فيزيائية 2020 الدورة الإستدراكية - التصحيحSohaula LBONINo ratings yet
- Bac2013S ADocument3 pagesBac2013S AOthmane MadaniNo ratings yet
- Bac2013M A PDFDocument5 pagesBac2013M A PDFOthmane MadaniNo ratings yet
- Devoir3 SVTDocument4 pagesDevoir3 SVTSiMo ElbNo ratings yet
- تمرين النقاوة للوحدة 4Document2 pagesتمرين النقاوة للوحدة 4essadikine anass67% (6)
- 02-مراقبة تطور جملة كيميائيةDocument26 pages02-مراقبة تطور جملة كيميائيةAbderrahman TalebNo ratings yet
- تمارين الوحدة الثالثة في البكالوريا بالحلDocument21 pagesتمارين الوحدة الثالثة في البكالوريا بالحلAbdou Islem100% (1)
- تصحيح إمتحان البكالوريا فيزياءمسلك علوم رياضية يونيو2011Document10 pagesتصحيح إمتحان البكالوريا فيزياءمسلك علوم رياضية يونيو2011saidphysique0% (1)
- Suj05r RevMaiDocument4 pagesSuj05r RevMaiitachi uchiwaNo ratings yet
- 5 1 08Document1 page5 1 08smjamal100% (1)
- Corpsmn18 PDFDocument13 pagesCorpsmn18 PDFnajiNo ratings yet
- الموضوع المقترح 03Document3 pagesالموضوع المقترح 03liltaoubaNo ratings yet
- U7b CoursDocument5 pagesU7b CoursAb DoNo ratings yet
- DevSol01M 2016Document3 pagesDevSol01M 2016rachidguessoum4No ratings yet
- Corppcr 21Document10 pagesCorppcr 21Zakaria AbdelhayNo ratings yet
- تطور الجمل الكهربائيةDocument9 pagesتطور الجمل الكهربائيةcol302011dzNo ratings yet
- تصحيح فيزياء الدورة العادية 2013 مسلك العلوم الفيزيائيةDocument3 pagesتصحيح فيزياء الدورة العادية 2013 مسلك العلوم الفيزيائيةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- إختبار الفصل -03- شعبة تر+ع ت - سنة 02 2022-2023Document4 pagesإختبار الفصل -03- شعبة تر+ع ت - سنة 02 2022-2023yanisyy979No ratings yet
- التحولات المقرونة بحمض-قاعدة و الكهرباءDocument2 pagesالتحولات المقرونة بحمض-قاعدة و الكهرباءYassine100% (1)
- فيزياء 2008مسلك علوم الحياة والأرضDocument9 pagesفيزياء 2008مسلك علوم الحياة والأرضالغزيزال الحسن EL GHZIZAL Hassane100% (11)
- تمارين بالحلDocument23 pagesتمارين بالحلchahinez Mecherrak0% (1)
- حل تمارين الدارة RC.pdf - Google DriveDocument1 pageحل تمارين الدارة RC.pdf - Google DriveOuail BeddalNo ratings yet
- 3AS-025 - موضوع اختبار تجريبي (1)Document16 pages3AS-025 - موضوع اختبار تجريبي (1)Rim BenguenabNo ratings yet
- ثالثة ع ت التبسيDocument2 pagesثالثة ع ت التبسيWissam GouasmiaNo ratings yet
- 6alfiziaa Oalkimiaa Alom Fiziaiia 2009 Aldora Alaadia Altshih 1Document5 pages6alfiziaa Oalkimiaa Alom Fiziaiia 2009 Aldora Alaadia Altshih 1Youssef YoussefNo ratings yet
- تصحيح استدراكية 09Document5 pagesتصحيح استدراكية 09ANASS ELYAAKOUBYNo ratings yet
- SV2008Document9 pagesSV2008lhajjiNo ratings yet
- 3AS U01 - E5 - Exercice 002 - تمرينDocument3 pages3AS U01 - E5 - Exercice 002 - تمرينRabah WinnrNo ratings yet
- 1- التطور التلقائي لمجموعة كيميائيةDocument6 pages1- التطور التلقائي لمجموعة كيميائيةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- Compo213 BrahimDocument4 pagesCompo213 BrahimAymen DjellalNo ratings yet
- 3as-005 - موضوع اختبار تجريبيDocument12 pages3as-005 - موضوع اختبار تجريبيRim BenguenabNo ratings yet
- ملخص الظواھر الكھربائیة فيزياء ثالثة ثانوي PDFDocument2 pagesملخص الظواھر الكھربائیة فيزياء ثالثة ثانوي PDFDilmi MohamedNo ratings yet
- ثالثة تر التبسيDocument2 pagesثالثة تر التبسيWissam GouasmiaNo ratings yet
- 1 - CoursDocument26 pages1 - CoursHB Mouh100% (1)
- Correction Des Exercies Lois de Newoton SM2014Document4 pagesCorrection Des Exercies Lois de Newoton SM2014yunuabou3No ratings yet
- امتحان تجريبي بكالوريا التعليم الثانوي دورة ماي 2014Document10 pagesامتحان تجريبي بكالوريا التعليم الثانوي دورة ماي 2014الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- Bac2013S BDocument5 pagesBac2013S BOthmane MadaniNo ratings yet
- 3AS U01 - E5 - Exercice 062 - تمرينDocument3 pages3AS U01 - E5 - Exercice 062 - تمرينRabah WinnrNo ratings yet
- S01SDocument2 pagesS01SOthmane MadaniNo ratings yet
- Corpsvtn 212Document7 pagesCorpsvtn 212Farid HaririNo ratings yet
- Resume PC 2bac 6Document3 pagesResume PC 2bac 6Mohammed KallouchiNo ratings yet
- تمارين في ثنائي القطب Rc 2Document10 pagesتمارين في ثنائي القطب Rc 2saidbrownNo ratings yet
- التحولات المقرونة بالتفاعلات حمض - قاعدةDocument12 pagesالتحولات المقرونة بالتفاعلات حمض - قاعدةibnolhaytam100% (2)
- Dzexams 2as Physique 1389674Document6 pagesDzexams 2as Physique 13896748d8cx2pqssNo ratings yet
- 1 PDFDocument14 pages1 PDFDjom AnaNo ratings yet
- 3asالفرض المحروس2017 اDocument1 page3asالفرض المحروس2017 اAmine ChaTtNo ratings yet
- تصحيح التمرين رقم 3 في درس المعايرة حمض قاعدة في مادة الفيزياء والكيمياء مستوى السنة الثانية بكالورياDocument2 pagesتصحيح التمرين رقم 3 في درس المعايرة حمض قاعدة في مادة الفيزياء والكيمياء مستوى السنة الثانية بكالورياZakaria ElhammoumiNo ratings yet
- عنوانDocument43 pagesعنوانbahaa oneproNo ratings yet
- باقة تمارين - الحلول الوحدة الأولى المفصلة للأستاذ فرقاني فارس - من رفع عقبة بن نافعDocument77 pagesباقة تمارين - الحلول الوحدة الأولى المفصلة للأستاذ فرقاني فارس - من رفع عقبة بن نافعmeriomaffNo ratings yet
- Mentouri AinMlilaDocument2 pagesMentouri AinMlilamohamed rezigNo ratings yet
- 1799693 فرض رقم 2 السنة الثانية ع فيزيائيةDocument2 pages1799693 فرض رقم 2 السنة الثانية ع فيزيائيةessadikine anassNo ratings yet
- 3as-021 - موضوع اختبار تجريبيDocument12 pages3as-021 - موضوع اختبار تجريبيRim BenguenabNo ratings yet
- الأقمار الاصطناعية و الكواكب : قوانين كيبلرDocument4 pagesالأقمار الاصطناعية و الكواكب : قوانين كيبلرالغزيزال الحسن EL GHZIZAL Hassane100% (13)
- 914383 الكهرباء ثنائي القطب Le Dipole RCDocument5 pages914383 الكهرباء ثنائي القطب Le Dipole RCالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- Tmarin Altholat Alkimiaiia Almqrona Baltfaalat HMDH Qaada Fi MhlolDocument3 pagesTmarin Altholat Alkimiaiia Almqrona Baltfaalat HMDH Qaada Fi Mhlolالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- قوانين نيوتن تمارينDocument2 pagesقوانين نيوتن تمارينالغزيزال الحسن EL GHZIZAL Hassane100% (1)
- تمارين في الأعمدة السنة الثانيةDocument1 pageتمارين في الأعمدة السنة الثانيةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- 2 التحولات التلقائية في الأعمدة وتحصيل الطاقةDocument8 pages2 التحولات التلقائية في الأعمدة وتحصيل الطاقةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- حالة توازن مجموعة كيميائيةDocument7 pagesحالة توازن مجموعة كيميائيةالغزيزال الحسن EL GHZIZAL Hassane100% (1)
- Exercices Acide Base 2bac1Document4 pagesExercices Acide Base 2bac1الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- DS-3 SPDocument3 pagesDS-3 SPالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- الموجات الكهرمغناطيسية تضمين الوسع: تمرين عن باكلوريا فرنسية2009Document2 pagesالموجات الكهرمغناطيسية تضمين الوسع: تمرين عن باكلوريا فرنسية2009الغزيزال الحسن EL GHZIZAL Hassane100% (1)
- تمرين الفرض الكتابي 2 الدورة الأولى السنة 2 ع ر 2: كيمياء أزهار الأورطنسية: النص الكامل عن باكلوريا فرنسيةDocument2 pagesتمرين الفرض الكتابي 2 الدورة الأولى السنة 2 ع ر 2: كيمياء أزهار الأورطنسية: النص الكامل عن باكلوريا فرنسيةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- الكهرباء ثنائي القطب Le Dipole RCDocument5 pagesالكهرباء ثنائي القطب Le Dipole RCالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- فرض كتابي رقم 2 التوازن الكيميائي،قياس المواصلة و الفيزياء النوويةDocument4 pagesفرض كتابي رقم 2 التوازن الكيميائي،قياس المواصلة و الفيزياء النوويةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- على طريق هنريش هرتز تمرين الحل في الموجات الكهرمغناطيسية وتضمينDocument7 pagesعلى طريق هنريش هرتز تمرين الحل في الموجات الكهرمغناطيسية وتضمينالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- التصوير الطبقي الشعاعي: تمرين مصحح في الفيزياء النووية من باكلوريا فرنسيةDocument5 pagesالتصوير الطبقي الشعاعي: تمرين مصحح في الفيزياء النووية من باكلوريا فرنسيةالغزيزال الحسن EL GHZIZAL Hassane100% (2)
- حالة توازن مجموعة كيميائيةDocument6 pagesحالة توازن مجموعة كيميائيةالغزيزال الحسن EL GHZIZAL Hassane100% (3)
- RC RLDocument17 pagesRC RLالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- 955265 ثنائي القطب RlDocument3 pages955265 ثنائي القطب Rlالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- فرض كتابي رقم 2 التوازن الكيميائي،قياس المواصلة و الفيزياء النوويةDocument4 pagesفرض كتابي رقم 2 التوازن الكيميائي،قياس المواصلة و الفيزياء النوويةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- Rl تمارين فيDocument3 pagesRl تمارين فيالغزيزال الحسن EL GHZIZAL Hassane100% (1)
- ثنائي القطب RlDocument2 pagesثنائي القطب Rlالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- 213Document2 pages213الغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- 955265 ثنائي القطب RlDocument3 pages955265 ثنائي القطب Rlالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- تمرين الفرض الكتابي 2 الدورة الأولى السنة 2 ع ر 2: كيمياء أزهار الأورطنسية: النص الكامل عن باكلوريا فرنسيةDocument2 pagesتمرين الفرض الكتابي 2 الدورة الأولى السنة 2 ع ر 2: كيمياء أزهار الأورطنسية: النص الكامل عن باكلوريا فرنسيةالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- 5 RCDocument8 pages5 RCmouyane mohamedNo ratings yet
- حالة توازن مجموعة كيميائيةDocument7 pagesحالة توازن مجموعة كيميائيةالغزيزال الحسن EL GHZIZAL Hassane100% (1)
- التصوير الطبقي الشعاعي: تمرين مصحح في الفيزياء النووية من باكلوريا فرنسيةDocument5 pagesالتصوير الطبقي الشعاعي: تمرين مصحح في الفيزياء النووية من باكلوريا فرنسيةالغزيزال الحسن EL GHZIZAL Hassane100% (2)
- 914383 الكهرباء ثنائي القطب Le Dipole RCDocument5 pages914383 الكهرباء ثنائي القطب Le Dipole RCالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- 955265 ثنائي القطب RlDocument3 pages955265 ثنائي القطب Rlالغزيزال الحسن EL GHZIZAL HassaneNo ratings yet
- Serie4 2009 2010Document3 pagesSerie4 2009 2010khalid el yacoubi67% (3)