Professional Documents
Culture Documents
Kipp Mende
Uploaded by
ridanormaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kipp Mende
Uploaded by
ridanormaCopyright:
Available Formats
Chem.
Educator 2011, 16, 295298
295
Gas Chemistry: A Microscale Kipp Apparatus
Metodija Najdoski
Institute of Chemistry, Faculty of Natural Sciences and Mathematics, Sts. Cyril and Methodius University, POB
162, Arhimedova 5, 1000 Skopje, Republic of Macedonia, metonajd@yahoo.com
Received January 12, 2011. Accepted July 16, 2011.
Abstract: A new version of microscale Kipp apparatus is proposed. The gas generating process is taking place in
a Beral pipets. The liquid reactant has to be inserted with a syringe into the pipet bulb. Depending on the required
gas volume that should be produced, two modifications are presented. This setup provides conditions for
generation of every gas that can be produced without heating in reaction between two liquids or solid and liquid
substances, or even between two gases. It is especially suitable for generation of nitric oxide, chlorine, hydrogen,
carbon dioxide, oxygen etc. The application of this apparatus is illustrated with several experiments. The idea
provides simple, safe and low cost experiments suitable for hands-on work.
Introduction
In the history of chemistry during XVII to XVIII century
with the work of many distinguished scientists the gas
chemistry was born and developed into inevitable, important
part of chemistry education for all ages.
Johann Baptista van Helmont (15791644) introduced the
word gas. Joseph Black (17281799) recognized the
existence of various airs (gases). Henry Cavendish (1731
1810) is a pioneer in the manipulation of gases and the one
who established the accurate composition of the air. Joseph
Priestley (17331804) discovered eight gases. Carl Wilhelm
Scheele (17421786) discovered oxygen, chlorine and several
other gases. Antoine Lavoisier (17431794) changed the
course of chemistry with his oxygen theory. In the 19th
century, the Dutch pharmacist Petrus Johannes Kipp (1808
1864) gave a special contribution to the gas chemistry with his
invention, the well-known Kipp apparatus. It was first
commercially produced in 1862, and since than till today this
apparatus has been frequently used in chemistry
demonstrations for generating hydrogen, acetylene, hydrogen
sulfide, and most often for generating carbon dioxide. Its use is
limited to gases that are generated by reaction between solid
and liquid reactant. The reactions between two liquids that are
exploited for gas generating are usually performed in apparatus
consisted of a Wrtz flask (or test tube) and a separatory
funnel or a syringe [1].
Another eminent chemist has deserved a credit for his
contribution to the gas chemistry especially in the field of
microscale gas chemistry. Professor Hubert Alyea introduced
gas generation with syringes in 1992 [2]. Since then, many
educators have presented their own way of making gases. The
prominent Professor Viktor Obendrauf from Pedagogical
Academy, Graz, Austria, has proposed numerous methods [3
7] for gas generation in a test tube using 2 mL syringe for
introducing liquid reactant and 20 mL syringe for collecting
the produced gas. Other prominent educator of our time,
Professor Bruce Mattson from Creighton University; Omaha,
Nebraska, also has given his contribution in numerous gas
generation methods inspired by Hubert Alyea article [2]. In
this area he has given a significant contribution with a great
number of articles in the journal Chem 13 News and his
Internet page [8]. He describes several methods with 50 to 60
mL syringes. One of them is the In-Syringe method with
general strategy of reaction of two substances in 60 mL
syringe where the limiting reagent is always used in a solid
form, placed in a small vial cap into the syringe barrel. Other
chemistry educators have given their contribution to the
development of apparatuses and methods in the field of
Microscale gas chemistry as well [912].
This article gives a contribution to the microscale gas
generation. In contrast of Obendrauf`s method that provides
about 20 mL of gas and Mattson`s methods that provide about
60 mL. The proposed method provides several mL of gas.
Experimental
Techniques of a gas generation with a microscale Kipp
apparatus. The design of the microscale Kipp apparatus is shown in
Figure 1.II and its modification in Figure 1.III. It consists of a Beral
pipet (3.5 mL) with shortened stem (a), 12 100 mm test tube (b),
Beral pipet (3.5 mL) with cut bulb and shortened stem (c), a stand
(e.g., made of gypsum or by drilling a hole in the center of a large
rubber stopper) (d), 1 mL insulin syringe (e) and a rubber stopper with
2 holes (f). In addition to this, a syringe (210 mL) for gas
manipulation is required.
Depending on the available rubber stoppers and the size of the
Beral pipets test tubes with different diameters can be used for
assembling the microscale Kipp apparatus. The functionality of the
parts is denoted by the same marks on the drawing of the original
Kipp apparatus and the microscale versions. The reaction for gas
generation takes place in the pipet bulb (Figure 1.IIa).
When the gas is generated by reaction between two liquids, the
plastic pipet is previously filled with liquid as it is shown in Figure 2.
The other liquid reagent is introduced in the pipets bulb with a
syringe. The produced gas is displacing the liquid which goes through
the stem in the test tube (Figure 1.IIb) and if there is enough liquid
than it goes in the reservoir (Figure 1.IIc). The used Beral pipets have
volume of 3.5 mL. When larger volume of gas is needed it might be
better to perform these experiments with pipets with large capacity
(5.8 mL; 15 mL or 24 mL).
The modification shown in Figure 1.III is simpler apparatus that is
preferably used in case when the required gas volume is equal or less
then the pipet volume (3.5 mL). The proposed microscale Kipp
apparatus (Figure 1.II) has no significant capacity for the liquid, but is
more educational in the teaching of construction and use of genuine
2011 The Chemical Educator, S1430-4171(11)12396-5, Published 10/31/2011, 10.1333/s00897112396a, 16110295.pdf
296
Chem. Educator, Vol. 16, 2011
Metodija Najdoski
test tube and if there is no rubber stopper it spreads in the surrounding
air. Instead of spreading out, if one uses the mentioned apparatus, the
gas is collected in the test tube increasing the pressure and displacing
the liquid into reservoir (Figure 1.IIc). Another way of avoiding this
problem with the gas spreading in the surrounding air (due to the low
reaction rate) is to add another liquid (solution), into the test tube that
reacts with the solution that comes from the pipet and terminates the
reaction of the gas production.
A) Generation of gas in reaction between liquids
Figure 1. Kipp apparatuses: I) Original, II) Microscale with reservoir,
III) Microscale simple modification.
Generation of NO. Fill a Beral pipet (in a position as it is shown in
Figure 2) with aqueous solution of FeSO4 prepared by dissolving 1.5 g
of FeSO47H2O in 30 mL 1520 % HCl. There should be no air
bubbles in the pipet. If there is some, invert the pipet upside down
with a bended stem, with the end in the same solution and by
squeezing, the air is pushed out and by releasing the pipet bulb is
filled with the solution.
Assemble the microscale Kipp apparatus with 1 mL of 10 % NaOH
in the test tube and fill insulin syringe with 0.1 mL of saturated
aqueous solution of NaNO2. Prepare one 5 mL syringe with needle
and a beaker with water and acid-base indicator. Insert the needle of
the insulin syringe in the pipet bulb and carefully add small amount of
solution of NaNO2. Sodium nitrite reacts with Fe2+ and colorless gas
(NO) is obtained and the solution changes the color to black according
to equation 1:
Fe2+(aq) + 2H3O+(aq) + NO2(aq)
NO(g) + Fe3+(aq) + 3H2O(l)
Figure 2. Filling the plastic pipet with a solution.
Kipp apparatus and allows collecting part of the gas into the test tube,
which in the other apparatus (Figure 1.III) will be spread into the air.
This may allow avoiding use of fume hood when the gas is toxic or
with unpleasant odor. Regarding this, one can always use the simpler
modification when there is no need of explanation to genuine Kipp
apparatus.
When gas generation that involves water insoluble solid substance
as reactant is required, then the pipet bulb should be horizontally cut
with scalpel (see Figure 3), enough to provide introducing (with
forceps) small lumps (grains) of reactant (see Figure 4). The cut
should be closed with an isolation tape or even better with isolation
tape. In some cases, when the gas is not soluble in water, the pipet is
filled with water or inert liquid to provide gas collecting in absence of
air. The water dilutes the liquid reactant that is introduced with a
syringe and for this reason the needle should be inserted closer to the
solid substance.
In both kind of experiments (with liquids or liquid-solid) the gas is
collected in an isolated space that provides relatively high purity of
the gases (besides water vapor). The technique is similar to collecting
gas under water. This is the way to avoid generating gas mixture with
air which is common when the gas is generated in a presence of air,
takes time and chemicals to collect a pure gas. This is especially
important when the generated gas is toxic or with bad odor.
In some experiments, when there is no need for generation of a
pure gas (with moisture), the syringe of 1 mL can be substituted with
5 or 10 mL syringe and the same syringe can be used for introducing
the liquid reactant and for collecting the produced gas by drawing it
and adding new portion of the liquid reactant again, if needed. This
technique with inserting reactant in and drawing out the gas product
that is toxic or with bad odor should be performed with the apparatus
shown in Figure 1.II. After repeating the cycle: adding liquid reactant
drawing the gas, the reaction of gas generation continues in the test
tube, in the liquid. This means that part of the gas is evolved into the
(1)
After the pipet bulb has approximately 23 mL of a colorless gas,
insert the needle of the 5 mL syringe and withdraw the gas (no more
than 2 mL) into the syringe and then close the syringe with a syringe
stopper [19]. Note the color of the gas and then draw air or better
1 mL oxygen, into the syringe. A reaction of NO and O2 takes place:
2NO(g) + O2(g) 2NO2(g)
(2)
Note the color change. The product of the reaction is brown gas
due to the equilibrium between NO2 (brown) and N2O4 (colorless).
Next, in the same syringe, draw some water with acid-base indicator
that changes color in acid media.
2NO2(g) + H2O(l) HNO3(aq) + HNO2(aq)
(3)
Note the changes. NO2 reacts with water producing a mixture of
acids, which decreases the gas pressure in the syringe and as a result
air is getting into the syringe and bubbles can be noticed.
One can collect more than 5 mL NO using cycle technique with
adding liquid reactant and drawing the gas in the syringe. In this case
NaOH(aq) should be avoided in the test tube. With 10 mL syringe
filled with 0.5 mL NaNO2(aq) one can collect 10 mL NO.
Warning: NO is toxic. Avoid breathing this gas. This experiment
should be performed under a fume hood. Due to the corrosive nature
of acids and alkaline solutions a special care should be taken (wear
rubber gloves and safety goggles).
Generation of Oxygen. Fill a Beral pipet with 6 % hydrogen
peroxide solution and assemble the microscale Kipp apparatus. Fill 1
mL syringe with 0.5 mL of 23 % solution of potassium
permanganate in 2 mol/dm3 H2SO4. Attach a needle with a wide
diameter of the hole on the syringes Luer-Lok connector and insert it
into the pipet bulb. After that, slowly add the potassium permanganate
solution into the pipet bulb. Oxygen generation is described with the
equation 4:
5H2O2(aq) + 2KMnO4(aq) + 3H2SO4(aq)
5O2(g) + 2MnSO4(aq) + K2SO4(aq) + 8H2O(l)
2011 The Chemical Educator, S1430-4171(11)12396-5, Published 10/31/2011, 10.1333/s00897112396a, 16110295.pdf
(4)
Gas Chemistry: A Microscale Kipp Apparatus
Chem. Educator, Vol. 16, 2011
297
Approximately 2 mL can be obtained. The solution that goes into
the test tube reacts with NaOH and becomes alkaline again, which
stops chlorine to be spread in the surrounding air.
Draw 2 mL diluted solution of potassium iodide (with few drops of
starch solution (colloidal system, as an indicator) that were
previously added) in 5 mL syringe. Then, attach a needle on the
syringes Luer-Lok connector and insert it into the pipet bulb and
draw some Cl2. Close the syringe with syringe stopper and shake it.
Note the color change due to the reaction shown with equation 7:
Cl2(g) + 2I(aq) I2(s) + 2Cl(aq)
(7)
The color change into deep blue is due to the formation of iodine
starch compound.
Warning: Chlorine is toxic. Avoid breathing this gas. This
experiment should be performed under a fume hood. Due to the
corrosive nature of acids and alkaline solutions a special care should
be taken (wear rubber gloves and safety goggles). The bleaching
solution may cause irritation. Avoid contact with eyes, skin, and
clothes. Wash hands after handling.
Figure 3. Cutting the plastic pipet.
B) Generation of gases in reaction of liquid and solid
Figure 4. Inserting Zn granules into the plastic pipet.
The obtained oxygen can be used for reaction with NO or H2 etc.
Warning: Hydrogen peroxide solution may cause irritation. Avoid
contact with eyes, skin, and clothes. Wash thoroughly after handling.
Keep container closed.
Generation of SO2. Fill a plastic pipet with saturated solution of
Na2SO3 or Na2S2O5. Place 1 mL of 10 % NaOH aqueous solution into
the test tube and assemble a microscale Kipp apparatus. Draw 0.2 mL
concentrated sulfuric acid into insulin syringe. Attach a needle (with a
wide diameter of the hole) on the syringes Luer-Lok connector and
insert it into the pipet bulb. Add slowly sulfuric acid in small portions.
The acid reacts with sulfite ions and sulfur dioxide is obtained as
shown in the equation 5:
Na2SO3(aq) + H2SO4(l) SO2(g) + Na2SO4(aq) + H2O(l) (5)
Draw 2 mL very diluted solution of methylene blue in one 5 mL
syringe. Attach a needle on the syringes Luer-Lok connector and
insert it into the pipet bulb and withdraw some amount (~ 3 mL) of
SO2. Close the syringe with a syringe stopper [19] and shake it. The
solution of methylene blue will be decolorized due to the reduction
properties of SO2.
Warning: Due to the corrosive nature of acids and alkaline
solutions a special care should be taken (wear rubber gloves and
safety goggles).
Generation of Cl2. Fill a Beral pipet with domestic bleaching
solution (20 % sodium hypochlorite). Add 1 mL of 10 % NaOH
aqueous solution into the test tube and assemble a microscale Kipp
apparatus. Draw 0.5 mL concentrated hydrochloric acid in 2 mL
syringe and attach a needle (with a wide diameter of the hole) to the
syringes Luer-Lok connector and insert it into the pipet bulb. Slowly
add the acid into the bulb. The acid reacts with ClO ions and
generates chlorine according to equation 6:
ClO(aq) + 2HCl(aq) Cl2(g) + Cl(aq) + H2O(l)
Generation of H2. Make a small horizontal cut on the pipet bulb
with a scalpel and add two granules of zinc or ~7 mm long Mg strip.
Use an isolation tape to close the cut of the pipet. Fill the pipet with
water and assemble a microscale Kipp apparatus. Fill one 2 mL
syringe with 1 mL concentrated hydrochloric acid. Set the needle on
the syringe and insert it into the pipet bulb closer to the metal. Slowly
add the acid as close as possible to the zinc granules.
After the gas is collected (see Figure 5) fill one 5 mL syringe with
2 mL of hydrogen and 2 mL chlorine according to the previous
experiment. In the fume hood, behind a protective door irradiate the
gas mixture by a photo flash. This does not work with all kinds of
photo flashes because some of them do not provide enough energy for
the photocatalytic process that is followed by an explosion that
generates HCl gas.
The generated hydrogen can be used for other reactions (with
oxygen, reduction of heated CuO in a tube or a test tube etc.), as well.
Warning: Chlorine is toxic. Avoid breathing this gas. This
experiment should be performed under a fume hood. Due to the
corrosive nature of acids a special care should be taken (wear rubber
gloves and safety goggles).
Generation of O2. Make a small horizontal cut in a Beral pipet bulb
with a scalpel. Add a granule made of mixture of MnO2 and cement
1:1 [3]. Use an isolation tape to close the cut of the pipet. Fill the pipet
with water and assemble a microscale Kipp apparatus. Fill one 1 mL
syringe with 1:1 solution of hydrogen peroxide and water. Set a
needle on the syringe and insert it into the pipet bulb. Add the
peroxide solution as close as to the granule. Around 3 mL of gas can
be obtained. Fill one syringe with 1 mL of oxygen. Using the
previously described experiment generate NO and with the same
syringe take 2 mL of NO. A reaction with color change occurs:
NO(g) + O2(g) 2NO2(g)
(8)
Generation of H2S. Insert one grain of pyrite or FeS into the pipet
bulb and close the cut with isolation tape. Place 2 mL of 10 % NaOH
aqueous solution into the test tube and assemble a microscale Kipp
apparatus. Draw 0.5 mL hydrochloric acid (the one for domestic use)
in 1 mL syringe. Attach a needle on it and insert it into the pipet bulb
and add several drops of the acid on the grain surface. The following
reaction occurs:
FeS(aq) + 2HCl(aq) H2S(g) + FeCl2(aq)
(6)
2011 The Chemical Educator, S1430-4171(11)12396-5, Published 10/31/2011, 10.1333/s00897112396a, 16110295.pdf
(9)
298
Chem. Educator, Vol. 16, 2011
Metodija Najdoski
into the test tube reacts with NaOH, becomes alkaline and stops
hydrogen sulfide from spreading in the surrounding air. At the end of
the experiment the excess of the H2S(g) can be eliminated by adding
NaOH(aq) with syringe through the same needle used for drawing out
H2S(g).
Due to the toxicity and the bad smell on rotten eggs of H2S, the
microscale Kipp apparatus is especially convenient. It allows
controlled generation of small volume of the gas which is usually
enough for performing some experiments.
Warning: H2S is toxic. Avoid breathing this gas. This experiment
should be performed under a fume hood. Due to the corrosive nature
of acids and alkaline solutions a special care should be taken (wear
rubber gloves and safety goggles). The solutions that contain Cd2+
may cause skin irritation. May be harmful if absorbed through the
skin. The solutions that contain Hg2+ cause severe eye and skin
irritation with possible burns. May be fatal if swallowed or if absorbed
through the skin. The solutions that contain Pb2+ may cause eye and
skin irritation. Prolonged exposure may result in skin burns and
ulcerations. Wear rubber gloves and safety goggles when handling
solutions of heavy metal salts.
Conclusions
Figure 5. Hydrogen generation.
Draw 1 mL of diluted aqueous solution that contains some of the
following cations: Cu2+, Cd2+, Hg2+, Pb2+, Sn2+, Bi3+, As3+ or Sb3+ in
several 5 mL syringes. Some of the solutions should be prepared with
addition of acid to prevent hydrolysis. Then attach a needle on the
syringe with the solution and insert it into the pipet bulb and draw out
some amount (~3 mL) of the produced H2S. Close the syringe with a
syringe stopper [13] and shake it. A colored precipitate occurs and the
reactions can be expressed with the following equations:
CuSO4(aq) + H2S(g) H2SO4(aq) + CuS(s)
black to brown precipitate
(10)
CdSO4(aq) + H2S(g) H2SO4(aq) + CdS(s)
yellow precipitate
(11)
Gas generation can be performed with a simple and safe
procedure, cheap equipment and available chemicals in a short
time at the microscale level. The proposed method could be
extended to generation of several other gases such as: SiH4,
PH3, HCN, C2H2, etc. The proposed novel microscale Kipp
apparatus for gas generation provides conditions for hands-on
experimentation. This idea makes the students active
participants in the educational process.
Supporting Materials. Safety Tips and Disposal
instructions
are
available
as
a
file
(http://dx.doi.org/10.1333/s00897112396a).
References and Notes
1.
Najdoski, M.; Petruevski, V. J. Chem. Educ., 2000, 77, 14471448.
2.
Alyea, H. N. J. Chem. Educ., 1992, 69, 65.
3.
Obendrauf, V. K. Lectures in Summer School, Ursprung-Salzburg,
2226 August, 2005.
(12)
4.
Obendrauf, V. K., Koehler-Kruetzfelddt, A. M. C., Chemical
Education Journal, 2003, 7, 14;
Pb(NO3)2(aq) + H2S(g) 2HNO3(aq) + PbS(s)
black precipitate
(13)
5.
http://chem.sci.utsunomiya-u.ac.jp/v7n2/angela/angela_viktor.html
(accessed January 2011).
6.
Obendrauf, V. K. Chemie&Schule, 1994, 4, 26.
2Bi(NO3)3(aq) + 3H2S(g) 6HNO3(aq) + Bi2S3(s)
black to brown precipitate
(14)
7.
Obendrauf, V. K. Chemie&Schule, 1999, 14, 1216.
8.
http://mattson.creighton.edu/Microscale_Gas_Chemistry.html
(accessed July 2011).
HgCl2(aq) + H2S(g) 2HCl(aq) + HgS(s)
black precipitate
SnCl2(aq) + H2S(g) 2HCl(aq) + SnS(s)
brown precipitate
(15)
2AsCl3(aq) + 3H2S(g) 6HCl(aq) + As2S3(s)
yellow precipitate
(16)
2SbCl3(aq) + 3H2S(g) 6HCl(aq) + Sb2S3(s)
orange precipitate
(17)
Note the color change. The experiment can be performed with
several syringes with different solutions and the H2S can be drawn
using just one needle by changing the syringes. The solution that goes
9.
Wang, J.; Lu, Z.; Zhao, C. J. Chem. Educ., 2003, 80, 181182.
10.
Kvittingen, L.; Verley, R. J. Chem. Educ., 2004, 81, 13391340.
11.
Eggen, P. O.; Kvittingen, L. J. Chem. Educ., 2004, 81, 13371338.
12. Choi, M. J. Chem. Educ., 2002, 79, 992993.
13. Syringe stoppers can be easily made of old syringe needles. Take a
needle to a metal part with a forceps and with fingers to the plastic
part. Heat the metal needle with flame of a micro burner and when it
is heated enough just pull the metal part away. The plastic part
should be heated carefully to melt and than just close the hole with
the aid of tweezers or pliers.
2011 The Chemical Educator, S1430-4171(11)12396-5, Published 10/31/2011, 10.1333/s00897112396a, 16110295.pdf
You might also like
- Recycling Used Oil Using Acetic AcidDocument27 pagesRecycling Used Oil Using Acetic Acidcumpio425428No ratings yet
- FW Solvent DeasphaltingDocument11 pagesFW Solvent Deasphaltingapminshull100% (1)
- Daftar PustakaDocument3 pagesDaftar PustakaridanormaNo ratings yet
- Appendiks C Spesifikasi PeralatanDocument2 pagesAppendiks C Spesifikasi PeralatanridanormaNo ratings yet
- Sulphur in PetroleumDocument26 pagesSulphur in PetroleumridanormaNo ratings yet
- Naphthenic Base Lube Oil Manufactured Via Hydrogenation Process Naphthenic Base Lube Oil Manufactured Via Hydrogenation ProcessDocument30 pagesNaphthenic Base Lube Oil Manufactured Via Hydrogenation Process Naphthenic Base Lube Oil Manufactured Via Hydrogenation ProcessDaniel DaiaNo ratings yet
- P138184 PDFDocument4 pagesP138184 PDFridanormaNo ratings yet
- 4 Girish ChitnisDocument22 pages4 Girish ChitnisridanormaNo ratings yet
- Ef 31752 e 0117 C 67585Document23 pagesEf 31752 e 0117 C 67585Rafanuñez RodriguezNo ratings yet
- United States Patent (19) : Powers Et Al. (11) Patent NumberDocument11 pagesUnited States Patent (19) : Powers Et Al. (11) Patent NumberridanormaNo ratings yet
- KomponenDocument15 pagesKomponenridanormaNo ratings yet
- Fuels and Lubricants Handbook Technology, Properties, Performance, and Testing - 0803120966 - ASTM International PDFDocument1,087 pagesFuels and Lubricants Handbook Technology, Properties, Performance, and Testing - 0803120966 - ASTM International PDFJerin James100% (1)
- Naphthenic Base Lube Oil Manufactured Via Hydrogenation Process Naphthenic Base Lube Oil Manufactured Via Hydrogenation ProcessDocument30 pagesNaphthenic Base Lube Oil Manufactured Via Hydrogenation Process Naphthenic Base Lube Oil Manufactured Via Hydrogenation ProcessDaniel DaiaNo ratings yet
- Ulllted States Patent (19) (11) Patent Number: 5,855,767: Powers Et Al. (45) Date of Patent: Jan. 5, 1999Document11 pagesUlllted States Patent (19) (11) Patent Number: 5,855,767: Powers Et Al. (45) Date of Patent: Jan. 5, 1999ridanormaNo ratings yet
- 02 Feedstocks & ProductsDocument133 pages02 Feedstocks & ProductsverdugrNo ratings yet
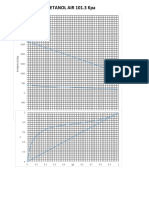
- Ethanol Air Phase Diagram ChartDocument1 pageEthanol Air Phase Diagram ChartridanormaNo ratings yet
- Us 2779713Document4 pagesUs 2779713ridanormaNo ratings yet
- Stream Properties Pompa Ke Separator F-120Document2 pagesStream Properties Pompa Ke Separator F-120ridanormaNo ratings yet
- Cha 4Document1 pageCha 4ridanormaNo ratings yet
- 1.0 IntroductionDocument2 pages1.0 IntroductionridanormaNo ratings yet
- Pre 2 30 July 2016 160731090013 PDFDocument284 pagesPre 2 30 July 2016 160731090013 PDFridanormaNo ratings yet
- Equilibrium Separation ColumnsDocument18 pagesEquilibrium Separation ColumnsluckshimiNo ratings yet
- Crystal EvapDocument7 pagesCrystal EvapridanormaNo ratings yet
- 4 Girish ChitnisDocument22 pages4 Girish ChitnisridanormaNo ratings yet
- 1471065083Document9 pages1471065083ridanormaNo ratings yet
- Proses Lubricating OilDocument5 pagesProses Lubricating OilridanormaNo ratings yet
- Proses Lubricating OilDocument5 pagesProses Lubricating OilridanormaNo ratings yet
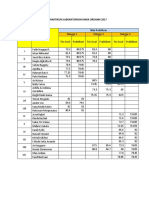
- Nilai Praktikum Laboratorium Kimia Organik 2017 Hari: KamisDocument1 pageNilai Praktikum Laboratorium Kimia Organik 2017 Hari: KamisridanormaNo ratings yet
- OSHA - OxygenDocument10 pagesOSHA - OxygenridanormaNo ratings yet
- Secant MethodDocument1 pageSecant MethodridanormaNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5782)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Water Treatment Math Formulas GuideDocument19 pagesWater Treatment Math Formulas GuidearjmandquestNo ratings yet
- Antimicrobials and AstringentsDocument52 pagesAntimicrobials and AstringentsApril Mergelle LapuzNo ratings yet
- Dedicated to Our ParentsDocument74 pagesDedicated to Our ParentsHassan Bin AliNo ratings yet
- Proper Cleaning Solutions FinalDocument4 pagesProper Cleaning Solutions Finalapi-555520296No ratings yet
- Comparative Study On Talahib CompositesDocument14 pagesComparative Study On Talahib CompositesKirsten Ignacio100% (1)
- CatalogDocument32 pagesCatalogPhan LuanNo ratings yet
- Effectiveness of Calamansi Extract as a Natural BleachDocument5 pagesEffectiveness of Calamansi Extract as a Natural Bleachadonis hamacNo ratings yet
- Modular Patient System (MPS) 3000 Bed Maintenance Manual: Important File in Your Maintenance RecordsDocument249 pagesModular Patient System (MPS) 3000 Bed Maintenance Manual: Important File in Your Maintenance RecordsEduardo Saul MendozaNo ratings yet
- BIOBASE Fume Hood FH (A) Series User Manual 202007Document37 pagesBIOBASE Fume Hood FH (A) Series User Manual 202007vmpazvNo ratings yet
- Dymax (E)Document2 pagesDymax (E)finetrend3186No ratings yet
- Lesson Plan in Integrated ScienceDocument6 pagesLesson Plan in Integrated Sciencerhyme_jiji50% (2)
- Silver Ionization Plant CatalogoueDocument3 pagesSilver Ionization Plant CatalogoueRavi Kumar VermaNo ratings yet
- Proposal Paper CE Sagarino Monte Adlaon With Chapter 4 and 5 PDFDocument48 pagesProposal Paper CE Sagarino Monte Adlaon With Chapter 4 and 5 PDFJansen OdronNo ratings yet
- Handouts in Rattan CraftDocument6 pagesHandouts in Rattan Crafteliver ladrilloNo ratings yet
- Mcqs On Bleaching of TeethDocument6 pagesMcqs On Bleaching of TeethIsaac NsengaNo ratings yet
- Stoichiometry I PostlabDocument4 pagesStoichiometry I Postlabapi-251760848No ratings yet
- Bleaching Methods of Cellulosic Textile MaterialDocument13 pagesBleaching Methods of Cellulosic Textile Materialshalini sharaffNo ratings yet
- Document Research PaperDocument6 pagesDocument Research PaperJustin jeav suicoNo ratings yet
- Texzyme BPL fungal cellulaseDocument16 pagesTexzyme BPL fungal cellulaseVidiaMumtazAlamryNo ratings yet
- Cadira Denim&Laundry 06 - 2020 PresentationDocument44 pagesCadira Denim&Laundry 06 - 2020 PresentationS.M. Sharif100% (1)
- Depex Bleach Regular Material Safety Data Sheet (MSDS) PDFDocument3 pagesDepex Bleach Regular Material Safety Data Sheet (MSDS) PDFLokman Mohd FadzilNo ratings yet
- Chlorine Dioxide: DPD Method Method 10126 0.04 To 5.00 MG/L Clo Powder PillowsDocument6 pagesChlorine Dioxide: DPD Method Method 10126 0.04 To 5.00 MG/L Clo Powder Pillowshiba JamalNo ratings yet
- 8.1-8.6 Redox ReactionsDocument65 pages8.1-8.6 Redox ReactionsberonelleNo ratings yet
- Types and Usage of Washing Machine and DryersDocument31 pagesTypes and Usage of Washing Machine and Dryersgrace.ganancialNo ratings yet
- General Chemistry 2 Online: Laboratory SafetyDocument14 pagesGeneral Chemistry 2 Online: Laboratory SafetyirfanNo ratings yet
- Laundry Linen Guest ClothesDocument196 pagesLaundry Linen Guest ClothesNickolodian AsuncionNo ratings yet
- Tumble Action Washer: Use & Care GuideDocument11 pagesTumble Action Washer: Use & Care GuidetheussatNo ratings yet
- Textile Auxiliaries: Zschimmer & SchwarzDocument54 pagesTextile Auxiliaries: Zschimmer & SchwarzRezoanul HaqueNo ratings yet
- Bleaching3 Konser2Document3 pagesBleaching3 Konser2akmalsatibiNo ratings yet
- TG No. 5 Bleaching and Finishing of RattanDocument12 pagesTG No. 5 Bleaching and Finishing of RattanAdolf NainggolanNo ratings yet