Professional Documents
Culture Documents
38jcis127 132
Uploaded by
secateOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
38jcis127 132
Uploaded by
secateCopyright:
Available Formats
Journal of Colloid and Interface Science 261 (2003) 127132
www.elsevier.com/locate/jcis
Effect of two-step solgel reaction on the mesoporous silica structure
Dae-Geun Choi and Seung-Man Yang
Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, 373-1 Guseong-dong, Yuseong-gu,
Daejeon 305-701, South Korea
Received 3 May 2002; accepted 3 January 2003
Abstract
In the present study, we investigated the effects of two-step solgel reaction by abrupt pH change on the SBA-15 and mesocellular
silica foams (MCF). Mesoporous silica was fabricated by using triblock copolymer templates (poly(ethylene oxide) and poly(propylene
oxide)). The prepared silica structure was characterized by X-ray diffraction, transmission electron microscopy, and N2 sorption experiment.
Specifically, we prepared SBA-15 with long-range two-dimensional hexagonal arrangement of 3 to 6-nm feature spacing and MCF with
larger pores of a few tens of nanometers. The pore size and ordering were influenced by pH change in a two-step solgel reaction and
the concentration of organic solvent. Although well-ordered hexagonal arrangement of mesopores was prevalent in acidic conditions, the
materials synthesized by a single-step reaction in neutral or basic conditions possessed gel-like structure without mesopores. However, the
present two-step reaction (low pH solgel reaction followed by high pH reaction) not only produced mesoporous materials but also provided
controllability of the pore size. In particular, mesoporous structures with pore sizes as large as those of MCF were successfully fabricated by
the two-step reaction without using organic swelling agents. As expected, when xylene was added as a swelling agent, the pore size increased
with the xylene/copolymer weight ratio.
2003 Elsevier Science (USA). All rights reserved.
Keywords: Two-step solgel reaction; Mesoporous silica; SBA-15; Mesocellular foam
1. Introduction
Mesoporous ceramic oxides with large specific surface
areas have potential applications in chemical and physical
processes, especially for chromatography and catalysis [1].
Since the discovery of MCM-41 in 1992 [2], many research
groups have focused their interests on mesoporous materials
[3]. MCM-41 is formed by a surfactant-assisted reaction
pathway as a result of the attractive electrostatic interaction
between a ceramic precursor and an ionic amphiphile. One
of the limitations of calcined MCM-41 materials prepared by
using cationic surfactants without additional treatment with
TEOS is their instability in water [4].
An alternative approach to mesoporous molecular sieves
is the exploitation of nonionic surfactants at low concentrations, which affords structural materials similar to MCM-41.
Pinnavaia and co-workers [5,6] used nonionic surfactants in
aqueous solutions to synthesize wormlike disordered mesoporous silica and alumina in neutral media assembled by
* Corresponding author.
E-mail address: smyang@mail.kaist.ac.kr (S.-M. Yang).
hydrogen-bonding interactions. Most of the ordered mesoporous silica materials organized with nonionic surfactant
species have been made under acidic conditions. Only several studies have been reported to synthesize ordered mesoporous materials under neutral or basic conditions in the
presence of fluoride anions [710]. Stucky and co-workers
showed that under the conditions where the hydrolysis proceeded rapidly, tetramethoxysilane (TMOS) was a preferable silica precursor to tetraethoxysilane (TEOS) for producing ordered mesostructures. They synthesized ordered
hexagonal mesoporous silica materials over a wide range
of pH by controlling the rate of hydrolysis relative to that
of condensation of silica species by use of fluoride and
TMOS [9].
It is well-known that triblock copolymers of poly(ethylene oxide) and poly(propylene oxide) in water form micelles
in which the core and shell are composed of poly(propylene
oxide) (PPO) blocks and poly(ethylene oxide) (PEO) blocks,
respectively [11]. At low pH, protonated PEO chains are
associated with cationic silica species through weak electrostatic interactions mediated by the negatively charged
chloride ions. Below the aqueous isoelectric point of sil-
0021-9797/03/$ see front matter 2003 Elsevier Science (USA). All rights reserved.
doi:10.1016/S0021-9797(03)00020-1
128
D.-G. Choi, S.-M. Yang / Journal of Colloid and Interface Science 261 (2003) 127132
ica in acidic media, cationic silica species will be present
as precursors, and the assembly might be expected to proceed through an intermediate form of (S0 H+ )(X I+ ). Nonionic block copolymers are an interesting class of structuredirecting agents whose self-assembly characteristics lead to
kinetically quenched structures. Block copolymers have the
advantage that they provide sacrificial templates whose microstructures can be tuned by adjusting solvent composition,
molecular weight, or copolymer architecture [12]. Moreover,
larger structural features are possible than that of low molecular weight surfactants and these are achieved at lower solution concentrations. Also, various template structures such
as bicontinuous cubic, hexagonal, or lamellar mesophases
can be produced by adding a small amount of a hydrophobic
swelling agent, such as butanol or xylene [13].
In the solgel route synthesis, a stepwise reaction scheme
has been undertaken to control the ratio of hydrolysis to condensation rates [14]. In general, the rate of hydrolysis is fast
compared to that of condensation in strong acidic conditions. Therefore, a well-ordered hexagonal arrangement of
mesopores is formed at low pH in acidic conditions. Meanwhile, in neutral or basic conditions ranging from pH 7 to
pH 9, the rate of condensation is faster than that of hydrolysis, and eventually the materials prepared by a single-step
reaction at high pH display gel-like structure without mesopores. It is hence an interesting attempt to synthesize ordered
mesoporous materials by a two-step solgel route at a lower
acidic pH followed by a higher pH. Up to now, however, a
two-step solgel reaction has not been applied for the fabrication of ordered mesoporous structure because its reaction mechanism has not been fully understood yet, and most
well-ordered silica structures have been achieved in strong
acidic conditions. This is the primary thrust of the present
study.
In this work, mesoporous silica was fabricated by using
triblock copolymer templates of PEOPPOPPO. Specifically, we prepared SBA-15 with long-range two-dimensional
hexagonal arrangement and mesocellular silica forms (MCF)
with larger pores by using xylene as a hydrophobic swelling
agent. Our goal here is to investigate the effects of a twostep solgel reaction induced by an abrupt pH change on
the SBA-15 and MCF structures. In the present two-step
reaction, the first step of the solgel reaction proceeded at
low pH and the second step at high pH. By doing this, ordered mesoporous materials were successfully synthesized
with pore sizes comparable to those of MCF.
2. Experimental
The triblock copolymer (EO20 PO70 EO20 (Pluronic P123),
MW = 5800) was obtained from BASF and used as received. Tetramethoxysilane (TMOS, Aldrich) and tetraethoxysilane (TEOS, Aldrich) in hydrochloric acid (HCl, Junsei)
were used as silica sources to prepare the silica mesophases.
Ordered silica mesostructures were synthesized by the meth-
od outlined in our recent publication [15], which is similar
to that reported by Zhao et al. [12]. First, 4.0 g of pluronic
P123 was dissolved in an acidic medium prepared with 30 g
of water and 120 g of 2 M HCl solution. Second, amounts
of xylene in the range of 0 to 8 g were added to the polymer solution, and the mixture was stirred for at least 1 h.
Then 8 g of TMOS (or TEOS) was added, and the resulting mixture was stirred at 308 K for 20 h for the synthesis
of general SBA-15 structure (H-0). Ammonium hydroxide
was used as a co-catalyst to induce stepwise pH change in
the two-step solgel reaction about 1 h after the addition of
TMOS. The mixture was aged at 353 K for 5 h. The solid
product was filtered, washed with water, and dried in an oven
for 4 h at 413 K. The triblock copolymer template was removed by solvent extraction (using ethanol and hydrochloric acid for 1 h) as well as by calcination in an electric furnace at 773 K for 6 h. The pore spacing and structure of the
prepared mesoporous materials were observed by using an
X-ray diffractometer (Rigaku, d/max-RC) under the strength
of 40 kV and 45 mA. A typical XRD analysis required several hours. The scattering data were collected in a continuous scan mode from 0.6 to 6 (2 ) with a sampling interval
of 0.01 at a scanning rate of 1 /min. A transmission electron microscope (TEM) (EM912, Carl Zeiss) was used to
examine the microstructure and grain size of the prepared
powders. To observe the individual grains, the aggregated
powders were dispersed in ethanol (0.5 wt%) and sonicated
for 20 min. The ultra-microtome method was used to observe the pore structure in an arbitrary orientation. Nitrogen
adsorption measurements at 77 K were performed on a volumetric adsorption analyzer (Micromeritics: ASAP 2010).
Before the measurement, the samples were outgassed for 4 h
at 473 K. The pore sizes, specific surface areas, and pore volumes were estimated using BrunauerEmmettTeller (BET)
and BarrettJoynerHalenda (BJH) desorption methods. Finally, SEM images of the prepared mesoporous silica were
taken to clarify the macroscale structure. The mass ratio of
P123, TMOS, and xylene was kept at 1:2:(02).
3. Results and discussion
3.1. Mesoporous structures formed in the absence of an
organic swelling solvent
First, we consider the SBA-15 samples (designated as
H-0) prepared in acidic conditions by a single-step solgel
route without adding ammonium hydroxide. As shown in
the TEM images of Figs. 1b and 1c, SBA-15 powder grains
are cylindrical-shaped and sized typically 200400 nm in
diameter and 13 m long. A well-ordered hexagonal array
of mesopores is observed for all calcined samples when the
electron beam is parallel to the major axis of the cylinder.
When the electron beam is perpendicular to the major axis,
the hexagonal arrays of the cylindrical pores are viewed
from the side, resulting in long channel-like images. The
D.-G. Choi, S.-M. Yang / Journal of Colloid and Interface Science 261 (2003) 127132
129
Fig. 2. TEM images of the mesoporous structures prepared at various pHs: (a) H-I prepared at pH 0.44 with the mass ratio of
NH4 OH/mixture = 0.033; (b) H-II prepared at pH 0.71 with the mass ratio
NH4 OH/mixture = 0.1; (c) H-III prepared at pH 4.54 with the mass ratio NH4 OH/mixture = 0.143; (d) H-IV prepared at pH 9.02 with the mass
ratio NH4 OH/mixture = 0.2.
Fig. 1. SEM (a) and TEM (b, c) images of the calcined SBA-15 (H-0:
prepared at pH 0.33 without NH4 OH).
two-dimensional hexagonal structure (p6mm) is therefore
confirmed. From the TEM images, the pore diameter was
estimated at 36 nm when TMOS was used as a silica
precursor. SEM images of the silica particles were taken
after calcinations of the materials in air at 773 K. In Fig. 1a,
the SEM image also revealed that SBA-15 has a hexagonal
cylinder shape with a uniform size of 200400 nm in
diameter and 13 m long.
As mentioned earlier, we prepared SBA-15 materials
by a two-step solgel route to examine the effect of pH
change on their microstructures. About 1 h after the addition
of TMOS to the templating polymer solution, ammonium
hydroxide was added as a co-catalyst. In Fig. 2, the TEM
images of the samples (designated as H-I, H-II, H-III, and
H-IV) prepared at various pH are reproduced. As noted
from Fig. 2, a well-ordered hexagonal array of mesopores
is observed at low pH. However, as the pH increased
by adding ammonium hydroxide, the pore structure was
transformed from an ordered to a disordered state. The
structural regularity remained up to pH 4.54 disappeared
completely at pH 9.02.
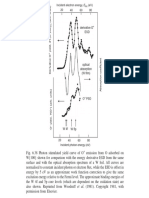
As shown in Fig. 3, the XRD results confirmed that the ordered hexagonal structure was formed in acidic conditions.
The XRD pattern shows well-resolved peaks that can be indexed as (100), (110), and (200) diffraction peaks associated
with p6mm ordered hexagonal structure. However, the XRD
peak intensity and resolutions were diminished gradually as
the pH increased. In Fig. 3, XRD patterns of the samples
(H-0, H-I, and H-II) prepared below the isoelectric point exhibited three clear peaks characteristic of a hexagonally or-
Fig. 3. XRD patterns of the samples prepared at different pHs.
dered structure. As in the case of the materials prepared at
high pH, relative intensities of (100), (110), and (200) peaks
reduced as the pH increased. It is because the structure was
transformed from an ordered to a disordered state as the pH
increased. Also, the X-ray diffraction results were consistent
130
D.-G. Choi, S.-M. Yang / Journal of Colloid and Interface Science 261 (2003) 127132
Fig. 4. XRD patterns of the samples (H-I) prepared at pH 0.44 as a function
of the addition time of NH4 OH.
with the TEM images. In Fig. 4, the XRD patterns were illustrated as a function of the addition time of ammonium
hydroxide, which was used as a co-catalyst to change the
pH. It can be seen from Fig. 4 that the well-ordered hexagonal array was formed as the first step reaction time increased
because the hexagonal structure was already formed during
the hydrolysis reaction of TMOS before pH change.
Nitrogen adsorption isotherms for the calcined samples
below the isoelectric point were similar to those reported
earlier [12,16] and displayed characteristics of good-quality
SBA-15. The isotherms of SBA-15 samples exhibited hysteresis loops with sharp adsorption and desorption branches.
The sharpness of the adsorption branches is indicative of a
narrow mesopore size distribution. Representative nitrogen
adsorption/desorption isotherms and the corresponding pore
size distributions are shown in Fig. 5. Pore size distributions
were analyzed by using the BJH desorption method. The silica particles (H-0) prepared without ammonium hydroxide
have an isotherm type IV with H1-type hysteresis that is typical of mesoporous materials with 1-D cylindrical channels.
The adsorption branches were located at relative pressures
in the range from 0.45 to 0.65. A narrow pore size distribution with a mean value of 38 is obtained from the BJH
desorption method. This material has a BET surface area
of 605 m2 /g and a pore volume of 0.49 cm3 /g, which are
listed in Table 1. These results were in good agreement with
those previously reported by Zhao et al. [12,16]. The materials (H-I) prepared with a low content of ammonium hydroxide but still below the isoelectric point showed similar
isotherms and exhibited pore sizes of 46 , BET surface area
of 615 m2 /g, and pore volume of 0.61 cm3 /g (Table 1). The
adsorption branches of H-I were located at relative pressures
in the range from 0.53 to 0.72, which were shifted toward
higher values relative to those for H-0. The positions of the
adsorption branches for SBA-15 shifted toward higher pressures as the pH increased. In addition, the pore size and BET
surface area increased with pH as shown in Table 1.
However, when the pH is above 7, the sample (H-IV)
displayed a different isotherm curve that was similar to
Fig. 5. Nitrogen adsorptiondesorption isotherms (top) and the pore size
distribution (bottom) for the calcined mesoporous silica particles prepared
by using the different amounts of NH4 OH.
that of MCF structure synthesized with an organic swelling
agent [10]. The pore size abruptly increased up to 200 ,
and the BET surface area decreased to 175 m2 /g. Thus,
the materials H-IV at this high pH exhibited a similar
isotherm curve and pore size as MCF structure. The main
difference between MCF and H-IV are the surface area
and the average pore sizes estimated from the nitrogen
adsorption and desorption. Usually, MCF materials have
surface area ranging from 580 to 900 m2 /g with spherical
pores [10], and SBA-15 has comparable surface area in
the range of 4001050 m2 /g [17]. The spherical pores
always have an ink bottle shape in which the spherical
bottles are connected to each other by smaller necks.
Therefore, the discrepancy in the average pore sizes between
the nitrogen adsorption and desorption is very large because
the pore size from adsorption reflects the size of the bottle,
and the pore size from desorption is associated with the
necks between pores. Our H-IV samples showed a narrow
difference between the adsorption and desorption isotherms
D.-G. Choi, S.-M. Yang / Journal of Colloid and Interface Science 261 (2003) 127132
131
Table 1
Structural properties of the selected samples estimated from nitrogen adsorption experiment
H-0
H-I
H-II
H-III
H-IV
pH
Volume ratio of NH4 OH/mixture
BET surface area (m2 /g)
Pore size () (BJH desorption)
Pore vol (cm3 /g)
0.33
0.44
0.71
4.54
9.02
0
0.03
0.10
0.14
0.20
605
615
615
732
175
38
46
48
69
200
0.49
0.61
0.57
1.22
0.91
Fig. 6. TEM images of the mesoporous structures prepared with different xylene contents. (a) R = 0.125; (b) R = 0.75; (c) R = 2.0. R is the mass ratio of
xylene/P123.
and had a relatively broad pore size distribution. The large
pore size and disordered structure of H-IV can be explained
as follows: At high pH above 7, condensation proceeds
rapidly compared to hydrolysis and thus silica nanoparticles
contain organic moieties (ethyl groups) due to incomplete
hydrolysis [18]. The residual ethyl groups lead to weaker
interactions between hydrophilic block copolymer and silica
nanoparticles, resulting in poorly ordered silica structures.
Therefore, structural density of H-IV is smaller than that
of a well-ordered silica structure. Also, an unreacted silica
precursor is able to penetrate the PPO core parts because of
weaker hydrophilicity, which leads to large pore size.
3.2. Mesoporous structures formed in the presence of an
organic swelling solvent
Fig. 7. XRD patterns of the mesoporous structures prepared with different
xylene contents. (a) R = 0.125; (b) R = 0.75; (c) R = 2.0.
The pore size of highly ordered hexagonal SBA-15 can be
controlled over the range 89300 by changing the amount
of TMB cosolvent in the reaction mixture [8,12,19]. We
synthesized MCF structure by using xylene as an organic
swelling agent. As expected, the pore size increased with
the xylene content and structure regularity was reduced as
shown in Fig. 6. XRD results reproduced in Fig. 7 were
very consistent with the TEM results. The hexagonal ordered
structure remained at low content of xylene, but structure
regularity was reduced as the xylene content increased.
Nitrogen adsorption isotherm for the calcined MCF
structure is shown in Fig. 8. MCF particles (Z-I) prepared
in acidic conditions without adding ammonium hydroxide
displayed the adsorption branches being located at relative
pressures in the range from 0.45 to 0.92. This is a broader
range than that of the SBA-15 particles. A narrow pore size
distribution with a mean value of 35 and 78 is obtained
from the BJH desorption and adsorption, respectively. The
BJH pore size was smaller than that estimated from the
TEM image (abut 1520 nm) because the BJH method
underestimated the size of the mesopores, neglecting the
effects of the curvature of the pore walls in ink bottle pore
structures [20]. Usually, the BJH method gives an accurate
estimation of pore size for cylindrical pores. However, the
pore size estimated by the BJH method reflects just the neck
of the pores in the ink bottle structure like MCF. These
materials had a BET surface area of 732 m2 /g and a pore
volume of 1.28 cm3 /g from the nitrogen adsorption test.
However, the sample Z-II prepared at pH 8.8 by a twostep solgel reaction showed behavior similar to sample HIV depicted in Fig. 5. The pore sizes were 153 and 186
from desorption and adsorption tests, respectively. The
132
D.-G. Choi, S.-M. Yang / Journal of Colloid and Interface Science 261 (2003) 127132
eral tens of nanometers), and the BET surface area was lower
than that of a typical SBA-15 or MCF.
Acknowledgments
This work has been supported partially by grants from
the Brain Korea 21 and IMT-2000 Projects. The authors
thank BASF for their kind donation of the Pluronic P123
block copolymer. The Korea Basic Science Institute is also
acknowledged for allowing us to use TEM (EM912, Carl
Zeiss).
References
Fig. 8. Nitrogen adsorptiondesorption isotherms.
BET surface area was 291 m2 /g, and the pore volume was
1.34 cm3 /g. In this case, the mesoporous silica structure
depended mainly on pH rather than swelling agent.
4. Summary
We prepared SBA-15 with a long-range two-dimensional
hexagonal arrangement and mesocellular silica forms (MCF)
with larger pores by using xylene as a hydrophobic swelling
agent. In particular, we investigated the effects of a twostep solgel reaction by abrupt pH change on the SBA-15
and MCF mesopore structures. As the amounts of ammonium hydroxide increased in the two-step solgel reaction,
the pore size was larger, and the structure regularity was reduced. The X-ray diffraction and nitrogen adsorption results
were consistent with the TEM images. The XRD peak intensity and resolution were diminished gradually with pH, and
the structure was transformed from an ordered to a disordered state. In the presence of xylene, the pore size increased
with the xylene content up to a certain saturation value above
which the pore size remained constant. The pore size was
strongly influenced by the pH and concentration of the organic solvent. In addition, mesoporous structures were successfully fabricated in basic or neutral conditions by the twostep reaction without using organic swelling agents. Their
pore size was large and similar to that of MCF (about sev-
[1] D. Antonelli, J. Ying, Curr. Opin. Colloid Interface Sci. 1 (1996) 523.
[2] C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck,
Nature 359 (1992) 710.
[3] C.G. Gltner, M. Antonietti, Adv. Mater. 9 (1997) 431.
[4] J.M. Kim, J.H. Kwak, S. Jim, R. Ryoo, J. Phys. Chem. 99 (1995)
16742.
[5] S.A. Bagshaw, E. Prouzet, T.J. Pinnavaia, Science 269 (1995) 1242.
[6] E. Prouzet, T.J. Pinnavaia, Angew. Chem. Int. Ed. Engl. 36 (1997) 516.
[7] A.C. Voegtlin, F. Ruch, J.L. Guth, J. Patarin, L. Huve, Microporous
Mater. 9 (1997) 95.
[8] P. Schmidt-Winkel, P. Yang, D.I. Margolese, B.F. Chmelka,
G.D. Stucky, Adv. Mater. 11 (1999) 303.
[9] J.M. Kim, Y.-J. Han, B.F. Chmelka, G.D. Stucky, Chem. Commun. 2
(2000) 437.
[10] P. Schmidt-Winkel, W.W. Lukens Jr., D. Zhao, P. Yang, B.F. Chmelka,
G.D. Stucky, J. Am. Chem. Soc. 121 (1999) 254.
[11] P. Alexandridis, T.A. Hatton, Colloids Surf. A 96 (1995) 1.
[12] D. Zhao, Q. Huo, J. Feng, B.F. Chmelka, G.D. Stucky, J. Am. Chem.
Soc. 120 (1998) 6024.
[13] P. Holmqvist, P. Alexandridis, B. Lindman, Macromolecules 30 (1997)
6788.
[14] W.L. Huang, K.M. Liang, S.R. Gu, J. Non-Cryst. Solids 258 (1994)
234.
[15] S. Wang, D.-G. Choi, S.-M. Yang, Adv. Mater. 14 (2002) 1311.
[16] D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka,
G.D. Stucky, Science 279 (1998) 548.
[17] M. Kruk, M. Jaroniec, C.H. Ko, R. Ryoo, Chem. Mater. 12 (2000)
1961.
[18] C.J. Brinker, G.W. Scherer, SolGel Science, Academic Press, San
Diego, 1992.
[19] P. Schmidt-Winkel, W.W. Lukens Jr., P. Yang, D.I. Margolese, J.S. Lettow, J.Y. Ying, G.D. Stucky, Chem. Mater. 12 (2000) 686.
[20] W.W. Lukens Jr., P. Schmidt-Winkel, J. Feng, G.D. Stucky, Langmuir 15 (1999) 5403.
You might also like
- AGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1Document8 pagesAGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1secateNo ratings yet
- ZnO nanostructures enhance optoelectronicsDocument3 pagesZnO nanostructures enhance optoelectronicssecateNo ratings yet
- Surfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodDocument4 pagesSurfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodsecateNo ratings yet
- Je5b00789 Si 001Document7 pagesJe5b00789 Si 001secateNo ratings yet
- PCCP Physical Chemistry Chemical Physics Accepted Manuscript Band AlignmentDocument11 pagesPCCP Physical Chemistry Chemical Physics Accepted Manuscript Band AlignmentsecateNo ratings yet
- Improved Temperature Compensation For in Situ Humic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water Types - Environmental Engineering ScienceDocument2 pagesImproved Temperature Compensation For in Situ Humic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water Types - Environmental Engineering SciencesecateNo ratings yet
- Improved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypesDocument4 pagesImproved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypessecateNo ratings yet
- Lambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundDocument1 pageLambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundsecateNo ratings yet
- Extraction of Titanium Dioxide from Ilmenite and Titaniferous Slag Using Fused Salt SolventsDocument6 pagesExtraction of Titanium Dioxide from Ilmenite and Titaniferous Slag Using Fused Salt SolventssecateNo ratings yet
- Arsenic in Drinking Water Problems and SolutionsDocument1 pageArsenic in Drinking Water Problems and SolutionssecateNo ratings yet
- E-Books: PublicationsDocument4 pagesE-Books: PublicationssecateNo ratings yet
- Copper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzerDocument2 pagesCopper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzersecateNo ratings yet
- Pancreatic JournalDocument4 pagesPancreatic JournalsecateNo ratings yet
- E-Books: PublicationsDocument4 pagesE-Books: PublicationssecateNo ratings yet
- Journal of Colloid and Interface ScienceDocument8 pagesJournal of Colloid and Interface SciencesecateNo ratings yet
- AN-v213 PDFDocument2 pagesAN-v213 PDFsecateNo ratings yet
- Two-Dimensional Vanadium-Doped ZnO Nanosheet Piezoelectric NanogeneratorDocument8 pagesTwo-Dimensional Vanadium-Doped ZnO Nanosheet Piezoelectric NanogeneratorsecateNo ratings yet
- Ab-416 3 enDocument20 pagesAb-416 3 ensecateNo ratings yet
- Quattro ESEM DatasheetDocument4 pagesQuattro ESEM DatasheetsecateNo ratings yet
- Meso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatteryDocument8 pagesMeso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatterysecateNo ratings yet
- Journal of Colloid and Interface ScienceDocument8 pagesJournal of Colloid and Interface SciencesecateNo ratings yet
- 10 1038@nenergy 2017 87Document9 pages10 1038@nenergy 2017 87secateNo ratings yet
- 2222 PDFDocument7 pages2222 PDFsecateNo ratings yet
- Dsc-Tga SimultaneoDocument1 pageDsc-Tga SimultaneosecateNo ratings yet
- Personalized PDF Catalog Catalogue Generated September 6, 2017Document4 pagesPersonalized PDF Catalog Catalogue Generated September 6, 2017secateNo ratings yet
- AaaaaaaaaaaaaaaaaaaaaaaaaaaaDocument1 pageAaaaaaaaaaaaaaaaaaaaaaaaaaaasecateNo ratings yet
- Personalized PDF Catalog Catalogue Generated September 6, 2017Document4 pagesPersonalized PDF Catalog Catalogue Generated September 6, 2017secateNo ratings yet
- Fig. 6.38Document1 pageFig. 6.38secateNo ratings yet
- File 1424717664Document8 pagesFile 1424717664secateNo ratings yet
- Sonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodsDocument6 pagesSonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodssecateNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- ABB 3HAC050988 AM Arc and Arc Sensor RW 6-En PDFDocument238 pagesABB 3HAC050988 AM Arc and Arc Sensor RW 6-En PDForefat1No ratings yet
- Electrical EngineerDocument3 pagesElectrical Engineer12343567890No ratings yet
- 2 Profile OMORIS - Presentation 2020-2Document20 pages2 Profile OMORIS - Presentation 2020-2lemuel bacsaNo ratings yet
- Product:: Electronic, 2 C #18 STR TC, PE Ins, OS, PVC JKT, CMDocument2 pagesProduct:: Electronic, 2 C #18 STR TC, PE Ins, OS, PVC JKT, CMAnonymous XYAPaxjbYNo ratings yet
- Design of PID controllersDocument4 pagesDesign of PID controllersFseha GetahunNo ratings yet
- Combustion Cat 2008Document32 pagesCombustion Cat 2008Miguel LinaresNo ratings yet
- Welcome To International Journal of Engineering Research and Development (IJERD)Document9 pagesWelcome To International Journal of Engineering Research and Development (IJERD)IJERDNo ratings yet
- Regenerative Medicine Manual ISSCA 2020 EnglishDocument21 pagesRegenerative Medicine Manual ISSCA 2020 EnglishDana MihutNo ratings yet
- DCI-2 Brief Spec-Rev01Document1 pageDCI-2 Brief Spec-Rev01jack allenNo ratings yet
- SSX Diaphragm: Truextent® Replacement Diaphragms For JBL and RADIANDocument2 pagesSSX Diaphragm: Truextent® Replacement Diaphragms For JBL and RADIANIvica IndjinNo ratings yet
- National Gypsum Purple Book Fire Rated Assemblies in Commercial Construction 1189979Document106 pagesNational Gypsum Purple Book Fire Rated Assemblies in Commercial Construction 1189979alvychuNo ratings yet
- Cosmic Freedom: David MolineauxDocument2 pagesCosmic Freedom: David Molineauxsalomon46No ratings yet
- Belden CatalogDocument24 pagesBelden CatalogMani MaranNo ratings yet
- Gene Regulation: Made By: Diana Alhazzaa Massah AlhazzaaDocument17 pagesGene Regulation: Made By: Diana Alhazzaa Massah AlhazzaaAmora HZzNo ratings yet
- Abb 60 PVS-TLDocument4 pagesAbb 60 PVS-TLNelson Jesus Calva HernandezNo ratings yet
- Shapes FlashcardsDocument5 pagesShapes FlashcardsHome Organising by JRNo ratings yet
- MATH8-Relations and Functions Worksheet AnswersDocument15 pagesMATH8-Relations and Functions Worksheet AnswersRhealyn Joy Narciso100% (2)
- Thank You For Taking The Week 3: Assignment 3. Week 3: Assignment 3Document3 pagesThank You For Taking The Week 3: Assignment 3. Week 3: Assignment 3DhivyaNo ratings yet
- L C R Circuit Series and Parallel1Document6 pagesL C R Circuit Series and Parallel1krishcvrNo ratings yet
- LogiquidsDocument2 pagesLogiquidsAloma FonsecaNo ratings yet
- H. Bateman, A. Erdélyi Et Al. - Higher Transcendental Functions 3 (1955, McGraw-Hill)Document310 pagesH. Bateman, A. Erdélyi Et Al. - Higher Transcendental Functions 3 (1955, McGraw-Hill)ITALO HERRERA MOYANo ratings yet
- MUCLecture 2021 10311889Document11 pagesMUCLecture 2021 10311889Ramon Angelo MendezNo ratings yet
- Tennis BiomechanicsDocument14 pagesTennis BiomechanicsΒασίλης Παπατσάς100% (1)
- 1999 - Seismic Soil Structure Interaction in Buildings - I Analytical Aspects PDFDocument13 pages1999 - Seismic Soil Structure Interaction in Buildings - I Analytical Aspects PDFCesar PugsioNo ratings yet
- Personality Types and Character TraitsDocument5 pagesPersonality Types and Character TraitspensleepeNo ratings yet
- POLIOMYELITISDocument26 pagesPOLIOMYELITISIzhra Margate100% (1)
- Mycbseguide: Cbse Class 10 Social Science Sample Paper - 08 (MCQ Based)Document10 pagesMycbseguide: Cbse Class 10 Social Science Sample Paper - 08 (MCQ Based)Abdul MuqsitNo ratings yet
- Year 5:: NUMBERS TO 1 000 000Document47 pagesYear 5:: NUMBERS TO 1 000 000Rusehaiza Bin Md DarusNo ratings yet
- Ridge regression biased estimates nonorthogonal problemsDocument14 pagesRidge regression biased estimates nonorthogonal problemsGHULAM MURTAZANo ratings yet