Professional Documents
Culture Documents
CHM13-2P 2011-2012 Syllabus
Uploaded by
Patrick ValdezOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHM13-2P 2011-2012 Syllabus
Uploaded by
Patrick ValdezCopyright:
Available Formats
MAPA INSTITUTE OF TECHNOLOGY
School of Chemical Engineering and Chemistry
VISION
The Mapua Institute of Technology shall be a global center of excellence in education by providing
instructions that are current in content and state-of-the-art in delivery; by engaging in cutting-edge, high-
impact research; and by aggressively taking on present-day global concerns.
MISSION
1. The Mapua Institute of Technology disseminates, generates, preserves and applies knowledge
in various fields of study.
2. The Institute, using the most effective and efficient means, provides its students with highly
relevant professional and advanced education in preparation for and furtherance of global
practice.
3. The Institute engages in research with high socio-economic impact and reports on the results of
such inquiries.
4. The Institute brings to bear humanitys vast store of knowledge on the problems of industry and
community in order to make the Philippines and the world a better place.
MISSION
EDUCATIONAL OBJECTIVES FOR BASIC STUDIES
1 2 3 4
1. To provide students with a solid foundation in mathematics, physics
and general chemistry and to apply knowledge to engineering,
architecture and other related disciplines.
2. To complement the technical training of the students with proficiency
in oral and written communication.
3. To instill in the students human values and cultural refinement
through the humanities and social sciences.
4. To inculcate high ethical standards in the students through its
integration in the learning activities.
COURSE SYLLABUS
1. Course Code: CHM13-2P
2. Course Title: GENERAL CHEMISTRY 3
3. Pre-requisite: CHM12-2, CHM12-2L
4. Co-requisite: None
5. Credit: 3 units
6. Course Description:
A continuation of CHM12-2, the course covers topics on chemical kinetics, chemical equilibria, ionic equilibria,
electrochemistry, nuclear chemistry, and detailed gravimetric analysis.
7. Program Outcomes and Relationship to Program Educational Objectives:
Course Title: Date Effective: Date Revised: Prepared by: Approved by:
GENERAL CHEMISTRY 3 3rd Quarter Page 1 of 4
(Lecture) SY 2010-2011 May 17, 2010
Edna J. Calderon Dean Luz L. Lozano
Program Outcomes Program
Educational
Objectives
The students are prepared to have achieved the program educational objectives by adopting a set 1 2 3 4
of program outcomes that the students are expected to have acquired by the time of their
graduation. These outcomes are as follows:
a an ability to apply knowledge of mathematics, science, and engineering
b an ability to design and conduct experiments, as well as to analyze and interpret from data
c an ability to design a system, component, or process to meet desired needs
d an ability to function on multidisciplinary teams
e an ability to identify, formulate, and solve engineering problems
f an understanding of professional and ethical responsibility
g an ability to communicate effectively
the broad education necessary to understand the impact of engineering solutions in the
h
global and societal context
I a recognition of the need for, and an ability to engage in life-long learning
j a knowledge of contemporary issues
an ability to use the techniques, skills, and modern engineering tools necessary for Addressed by
engineering practice some
k
professional
courses
8. Course Objectives and Relationship to Program Outcomes:
Course Objectives Program Outcomes
The students should be able to: a b c d e f g h i j k
1. To acquire and retain a basic, working knowledge of the fundamental
concepts taught which shall serve as groundwork for the subsequent
Chemistry courses for which this course is a pre-requisite.
2. To be able to relate the concepts learned to practical applications and
gain a general, positive appreciation of the importance of Chemistry
thus making its study a challenging endeavor.
3. To acquire specific values which are inherent in Chemistry as a
science, such as the preservation of good health and clean
environment through the control and prevention of air and water
pollution.
9. Course Coverage:
WEEK TOPIC METHODOLOGY ASSESSMENT
Orientation
Chemical Kinetics Lecture Homework / Seatwork
The Rate of a reaction Problem Solving Examination
1
Factors that Affect Reaction Rate
Concentration Versus Time Lecture Homework / Seatwork
Problem Solving Examination
Activation Energy; Temperature; and Catalysis Lecture Homework / Seatwork
Reaction Mechanism Problem Solving Examination
Practice Exercise 1
2 Chemical Equilibrium Lecture Homework / Seatwork
Basic Concepts Problem Solving Examination
Equilibrium Constant Expression and Value of K
Calculation of K
Exam No. 1: Diagnostic Exam (Coverage CHM11-2 and CHM12-2)
The Reaction Quotient Lecture Homework / Seatwork
Uses of the Equilibrium Constant Problem Solving Examination
3 Factors that Influence Equilibrium
Practice Exercise 2
Exam No. 2
Ionic Equilibria Lecture Homework / Seatwork
Review of Strong Electrolytes Problem Solving Examination
4 The Autoionization of Water
The Concepts of pH and pOH
The Ionization Constants for Weak Acids and Bases
Course Title: Date Effective: Date Revised: Prepared by: Approved by:
GENERAL CHEMISTRY 3 3rd Quarter Page 2 of 4
(Lecture) SY 2010-2011 May 17, 2010
Edna J. Calderon Dean Luz L. Lozano
Solvolysis Lecture Homework / Seatwork
Problem Solving Examination
The Solubility Product Principle Lecture Homework / Seatwork
Solubility Product Constants Problem Solving Examination
Uses of Solubility Product Constants
Fractional Precipitation
Practice Exercise 3
Exam No. 3
5
Electrochemistry Lecture Homework / Seatwork
Redox Reactions Problem Solving Examination
Electrolytic Cells Lecture Homework / Seatwork
Faradays Law Problem Solving Examination
Voltaic Cells Lecture Homework / Seatwork
6 Standard Cell Potentials Problem Solving Examination
Concentration Effects
Nuclear Chemistry Lecture Homework / Seatwork
Nuclear Stability and Radioactivity Problem Solving Examination
Nuclear Reactions
Rates of Decay Lecture Homework
Problem Solving Seatwork
7 Examination
Practice Exercise 4
Exam No. 4
Review on Exit Exam
Exit Exam
8 Gravimetric Methods of Analysis Lecture Homework / Seatwork
Review on Mole and Millimole Calculations Problem Solving Examination
Chemical Stoichiometry
Precipitation and Evolution Gravimetry Lecture Homework / Seatwork
Problem Solving Examination
9 Calculation of Results from Gravimetric Data (Pure Lecture Homework / Seatwork
Precipitates) Problem Solving Examination
Calculation of Results From Gravimetric Data Lecture Homework / Seatwork
(Mixtures of Precipitates) Problem Solving Examination
Practice Exercise 5
10 Exam No. 5
Review on Final Exam
11 Final Exam
10. Student Outcomes and Relationship to Course Objectives/ Program Outcomes:
Course Program Outcomes
Student Outcomes Objectives
1 2 3 a b c d e f g h i j K
Apply principles gained from the prerequisite
1
courses.
Perform basic chemical kinetics and chemical
2
equilibrium calculations
Apply equilibrium principles in systems of acids,
3
bases and salts
4 Perform electrochemical and nuclear calculations
Understand the concepts and be familiar with the
5 steps/techniques employed in gravimetric
methods of analysis
11. Contribution of Course to Meeting the Professional Component:
Basic Sciences and Mathematics - 95%
General Education - 5%
12. Textbook:
th
Whitten, Kenneth W., et. al., CHEMISTRY 9 ed, Brooks/Cole Cencage Learning 2010.
Course Title: Date Effective: Date Revised: Prepared by: Approved by:
GENERAL CHEMISTRY 3 3rd Quarter Page 3 of 4
(Lecture) SY 2010-2011 May 17, 2010
Edna J. Calderon Dean Luz L. Lozano
13. Course Evaluation:
Student performance will be rated in the following manner:
13.1 Examinations 50 %
13.2 Other Requirements 25 %
(Seatworks, Homeworks, etc.)
13.3 Final Examinations 25 %
TOTAL 100 %
The final grades shall correspond to the weighted average scores shown below:
Final Average Grade Average Grade
Below 70.00 5.00 83.01 86.00 2.00
70.00 73.00 3.00 86.01 90.00 1.75
73.01 76.00 2.75 90.01 93.00 1.50
76.01 80.00 2.50 93.01 96.00 1.25
80.01 83.00 2.25 96.01 100.00 1.00
14. Other Course Policies:
Attendance
According to CHED policy, total number of absences by the students should not be more than
20% of the total number of meetings or 9 hours for this two-unit course. Student incurring more than 9 hours
of unexcused absences automatically get a failing grade regardless of class standing.
Class Exercises, Learning Tasks, Quizzes
Learning tasks and other assigned works or projects are due at the beginning of the class periods of the
specified dates. Late assignments are not accepted. Quizzes are to be taken only on the dates announced/
specified. No special quizzes are given except for meritorious cases.
Language of Instruction
Lectures, discussions and class presentations will be in English.
Honor, Dress and Grooming Codes
Everybody has been instructed on the dress and grooming codes of the Institute. Everybody must commit to
abide by these codes.
For this course, the Honor Code is that there will be no plagiarizing on written work and no cheating on
examinations. Proper citation must be given to authors whose works were used in the process of developing
instructional materials and learning for this course. If a student is caught cheating on an exam, he/ she will be
given a zero mark for that particular exam. If a student is caught cheating for the second time, he/ she will be
referred to the Prefect of Student Affairs, the guidance Office and will be given a failing mark for the course.
Consultation Schedule
Consultation schedules with the professors are posted outside the ChE-Chm Faculty room and in the school
web-page (http://che-chm.mapua.edu.ph). It is recommended that the student first set an appointment to
confirm the instructors availability.
15. Other References:
Books
TH
a. Whitten, Kenneth W. et. al., GENERAL CHEMISTRY , 8 edition, Thomson Brooks/Cole
th
b. Silberberg, Martin S., CHEMISTRY: The Molecular Nature of Matter and Change. 4 ed, McGraw-Hill
th
c. Brown, Le May and Bursten, Chemistry: The General Science, 10 ed, Prentice-Hall International, Inc
TH
d. Chang, Raymund, Chemistry, 8 edition, McGraw-Hill
Course Materials Made Available
Course goals and instructional objectives
Course schedule for lectures and Exams
End of course self-assessment report
16. Committee Members:
Calderon, Edna J. Miranda, Marilyn A.
Cruz, Kathlia DC Ng, Josephine A.
Espiritu, Elizabeth S. Santos, Nanette D.
Martin, Marilen M. Velarde, Homer C.
Course Title: Date Effective: Date Revised: Prepared by: Approved by:
GENERAL CHEMISTRY 3 3rd Quarter Page 4 of 4
(Lecture) SY 2010-2011 May 17, 2010
Edna J. Calderon Dean Luz L. Lozano
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- HAZOPDocument75 pagesHAZOPPatrick ValdezNo ratings yet
- FM RS 08 02 Proposal Oral Examination v.1Document1 pageFM RS 08 02 Proposal Oral Examination v.1Patrick ValdezNo ratings yet
- Exercise 1 - Material BalanceDocument1 pageExercise 1 - Material BalancePatrick ValdezNo ratings yet
- FM RS 08 14 Proposal Billing Form v.1Document1 pageFM RS 08 14 Proposal Billing Form v.1Patrick ValdezNo ratings yet
- Taules TG Oxford PDFDocument39 pagesTaules TG Oxford PDFBaban BaidyaNo ratings yet
- AbstractDocument2 pagesAbstractPatrick ValdezNo ratings yet
- CHM13P Learning Task 5Document6 pagesCHM13P Learning Task 5Paolo Gochingco0% (3)
- MP2: Equations of State: Engr. Elisa G. EleazarDocument20 pagesMP2: Equations of State: Engr. Elisa G. EleazarPatrick ValdezNo ratings yet
- 211 1199 2 PB PDFDocument22 pages211 1199 2 PB PDFPatrick ValdezNo ratings yet
- Molecular Symmetry: Symmetry Element Symmetry Operation SymbolDocument6 pagesMolecular Symmetry: Symmetry Element Symmetry Operation SymbolPatrick ValdezNo ratings yet
- Particle Distribution of SoilDocument4 pagesParticle Distribution of SoilPatrick ValdezNo ratings yet
- MP3 - Equations of StateDocument8 pagesMP3 - Equations of StatePatrick ValdezNo ratings yet
- Academic HandbookDocument560 pagesAcademic HandbookPatrick Valdez100% (1)
- Problem 8 7bDocument24 pagesProblem 8 7bPatrick ValdezNo ratings yet
- Extended SurfacesDocument17 pagesExtended SurfacesPatrick ValdezNo ratings yet
- Separation Processes Problem Set 1Document1 pageSeparation Processes Problem Set 1Patrick ValdezNo ratings yet
- Exp 3Document4 pagesExp 3Patrick ValdezNo ratings yet
- Deviance Theory PaperDocument2 pagesDeviance Theory PaperPatrick ValdezNo ratings yet
- Quiz 5 - Mec30Document2 pagesQuiz 5 - Mec30Patrick ValdezNo ratings yet
- Equations 2Document8 pagesEquations 2Patrick ValdezNo ratings yet
- MAPUA FSM Drill 4 - Finite State Machines Practice ProblemsDocument1 pageMAPUA FSM Drill 4 - Finite State Machines Practice ProblemsPatrick ValdezNo ratings yet
- Expt4 STD CalibDocument6 pagesExpt4 STD CalibPatrick ValdezNo ratings yet
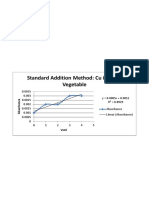
- Standard Addition Method: Cu in Leafy VegetableDocument1 pageStandard Addition Method: Cu in Leafy VegetablePatrick ValdezNo ratings yet
- 01 Intro To BioengDocument30 pages01 Intro To BioengPatrick ValdezNo ratings yet
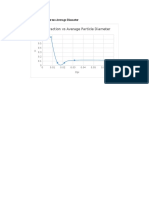
- Mass Fraction vs Average Particle Diameter ChartDocument8 pagesMass Fraction vs Average Particle Diameter ChartPatrick ValdezNo ratings yet
- Mass Fraction Vs Average Particle DiameterDocument2 pagesMass Fraction Vs Average Particle DiameterPatrick ValdezNo ratings yet
- Electrogravimetric Determination of Metals from MixturesDocument5 pagesElectrogravimetric Determination of Metals from MixturesPatrick ValdezNo ratings yet
- Electrogravimetric Determination of Metals from MixturesDocument5 pagesElectrogravimetric Determination of Metals from MixturesPatrick ValdezNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Teachers DiaryDocument9 pagesTeachers Diarytiwari_anunay16890% (2)
- Content Knowledge and Pedagogy: Respectfully SubmittedDocument17 pagesContent Knowledge and Pedagogy: Respectfully SubmittedCalventas Tualla Khaye JhayeNo ratings yet
- Boehler Et Al 2006 An Investigation of Medical Student Reactions To Feedback A Randomized Controlled TrialDocument4 pagesBoehler Et Al 2006 An Investigation of Medical Student Reactions To Feedback A Randomized Controlled Trialapi-264671444No ratings yet
- FALL 16 Course SyllabusDocument18 pagesFALL 16 Course SyllabussamNo ratings yet
- UdlDocument3 pagesUdlapi-309585191No ratings yet
- Using Telepresence to Support and Deliver LearningDocument13 pagesUsing Telepresence to Support and Deliver LearningMahaManthraNo ratings yet
- Deliberate Practice StrategiesDocument11 pagesDeliberate Practice StrategiesYOHANES EDWALDUSNo ratings yet
- Resume Making Workshop - IIT BombayDocument49 pagesResume Making Workshop - IIT BombayAnshul KhandelwalNo ratings yet
- Educational Technology 2Document31 pagesEducational Technology 2Ezekiel D. Rodriguez100% (2)
- Mini Lesson Passe ComposeDocument3 pagesMini Lesson Passe Composeapi-324919699No ratings yet
- Getting Into Harvard - The Long March From China To The Ivies - 1843Document16 pagesGetting Into Harvard - The Long March From China To The Ivies - 1843Biswajeet PattnaikNo ratings yet
- Spanking Children Controversy, Findings and New DirectionsDocument28 pagesSpanking Children Controversy, Findings and New DirectionsIle Pi100% (1)
- Barriers of Communication Lesson Plan for Grade 11Document5 pagesBarriers of Communication Lesson Plan for Grade 11Marie Javier0% (1)
- Islamization of The CurriculumDocument19 pagesIslamization of The Curriculumihateregistrationn100% (2)
- 11161+-+Art+10+-+TEACHERS+AS+FACILITATORSDocument10 pages11161+-+Art+10+-+TEACHERS+AS+FACILITATORSEL MEHDI AL AZIZINo ratings yet
- SyllabusDocument3 pagesSyllabusSean LoNo ratings yet
- Module 14 14Document2 pagesModule 14 14Fenny Lou CabanesNo ratings yet
- Erin Wittenberg: Professional SummaryDocument2 pagesErin Wittenberg: Professional SummaryErin WittenbergNo ratings yet
- Universiti Tunku Abdul Rahman (Utar) : Faculty of Accountancy and ManagementDocument6 pagesUniversiti Tunku Abdul Rahman (Utar) : Faculty of Accountancy and ManagementChee Wai KenNo ratings yet
- First AidDocument4 pagesFirst Aidapi-296874733No ratings yet
- Parent Welcome Letter1.Telicia - 2Document6 pagesParent Welcome Letter1.Telicia - 2tevil9555No ratings yet
- The Last Twenty-Five Years of CAASDocument15 pagesThe Last Twenty-Five Years of CAASmilosmouNo ratings yet
- Sight Singing in The Beginning High School ClassroomDocument16 pagesSight Singing in The Beginning High School ClassroomJoeEvelerNo ratings yet
- Power Mentoring Ensher en 5436 PDFDocument6 pagesPower Mentoring Ensher en 5436 PDFJoko HaryonoNo ratings yet
- Curriculum Map2Document4 pagesCurriculum Map2api-308717488No ratings yet
- cw2 - Interview - Epc 2403 Interview Questions 2Document5 pagescw2 - Interview - Epc 2403 Interview Questions 2api-376004495No ratings yet
- BUDT 758B Big Data - Syllabus-2016 - Gao & Gopal - 0Document4 pagesBUDT 758B Big Data - Syllabus-2016 - Gao & Gopal - 0pooh06No ratings yet
- McKinsey Problem Solving Test - PSTDocument28 pagesMcKinsey Problem Solving Test - PSTAmber Roberts33% (6)
- L2 - English Lesson FormatDocument21 pagesL2 - English Lesson FormatMajdoline Sadeddine0% (1)
- Sample Pa Ges CatalogueDocument16 pagesSample Pa Ges CatalogueEndi100% (2)