Professional Documents
Culture Documents
Mine Synthesis
Uploaded by
riskobinskoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mine Synthesis
Uploaded by
riskobinskoCopyright:
Available Formats
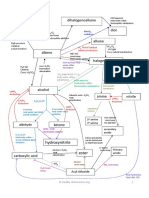
C. S. Marvel, Methylamine HydrochlorideOrganic Syntheses Coll. Vol.
I, pp347-350, 2nd Ed (1958) In a 5 liter round-bottomed flask, fitted with a stopper holding a condenser set for downward distillation and a thermomether which will extend well into the liquid, are placed 4 kg (3711 ml, 47-53 moles) of technical formaldehyde (35-40 percent; d 1.078 at 20C) and 2 kg (37 moles) of technical ammonium chloride. The mixture is heated on the steam bath until no more distillate comes over and then over a flame until the temperature of the solution reaches 104C. The temperature is held at this point until no more distillate comes over (four to six hours). The distillate, which consists of methylal (bp 42-43C), methylformate and water may be treated with NaOH solution to recover methylal and sodium formate. The contents of the reaction flask are cooled too room temp and the ammonium chloride which separates is filtered off. The mother liquor is concentrated on the steam bath under reduced pressure to 2500 ml, and again cooled to room temp, whereupon a second crop of ammonium chloride separates. The total recovery of ammonium chloride up to this point amounts to 780-815 grams. The mother liquor is again concentrated under reduced pressure until crystals begin to form on the surface of the solution (1400-1500 ml). It is then cooled to room temperature, and a first crop of methylamine hydrochloride, containing some ammonium chloride is obtained by filtering the cold solution. At this point 625-660 grams of crude product is obtained. The mother liquor is now concentrated under reduced pressure to about 1000 ml, and cooled, and a second crop of methylamine hydrochloride (170-190 grams) is then filtered off. This crop of crystals is washed with 250 cc of cold chloroform, and filtered to remove most of the dimethylamine hydrochloride which is present. After the washing, the product weighs 140-150 grams. The original mother liquor is then evaporated under reduced pressure, as far as possible, by heating on a steam bath, and the thick syrupy solution (about 350 ml) which remains is poured into a beaker and allowed to cool, with occasional stirring, in order to prevent the formation of a solid cake, and the crystals obtained are washed with 250 ml of cold chloroform, the solution is filtered yielding 55-65 grams of product. There is no advantage in further concentrating the mother liquor, which contains mostly tetramethylmethylenediamine hydrochloride, but no trimethylamine hydrochloride. The total yield of methylamine hydrochloride is 830-850 grams. The product contains water, ammonium chloride and some dimethylamine hydrochloride. In order to obtain a pure product, the impure methylamine hydrochloride is
recrystallized from absolute ethanol (solubility 0.6g/100ml at 15C), or preferably butyl alcohol (even less soluble). The recovery of ammonium chloride amounts to 100-150 grams, making the total recovery 850-950 grams. The yield of recrystallized methylamine hydrochloride is 600-750 grams (45-51 percent of theory, based on the used up ammonium chloride). A standard run, from 250 grams ammonium chloride and 500g 37% formaldehyde (containing 15% methanol), gives 100-134 grams methylamine hydrochloride, 27 grams dimethylamine hydrochloride and 81 grams of recovered ammonium chloride. The distillate contains methylal (formaldehyde dimethyl acetal) and methyl formate, which after treatment with NaOH can yield 25g of sodium formate and 30 grams of methylal, as the compound cannot be separated by fractional distillation, neutralization is the way to go. Ammonium chloride is very sparingly soluble in a concentrated solution of methylammonium chloride, making the separation of the compounds pretty sharp. H. I. Jones, The Preparation of Methylamine, J. Am. Chem. Soc. (?), pp1411-1515 (1918) 50 grams of ammonium chloride and 300g of 40% formaline solution were slowly warmed together under vacuum, and soon a rapid evolution of carbon dioxide began. The solution was refluxed at a 20 mm pressure for four hours, until no more carbon dioxide was given off, as tested with barium hydroxide solution. The reaction product was then distilled in a vacuum, 2 hours being consumed in the distillation. As soon as the residue from the distillation was cold, the ammonium chloride which had crystallized out was filtered off with suction, and the filtrate further concentrated by evaporation and again cooled, allowing further ammonium chloride to crystallize. As Werner has pointed out [1], the separation of ammonium chloride and methylammonium chloride is pretty sharp, and the crystals are so different that it is easy to see if one is contaminated with the other. The collected ammonium chloride wighed 7.9 grams (0.17 mol). The mother liquor left was evaporated until crystals began to form on the surface, and was set aside to cool, and the first crop of methylammonium chloride was collected with suction. Further evaporation gave additional crops. In all four crops that were obtained, which were freed from dimethylamine hydrochloride by washing with chloroform, 27.2 grams of dimethylamine hydrochloride (0.51 mol)was obtained. The distillate weighed 37.3 grams, the residue which didn't crystallize weighed 326 grams, and the total
yield of methylamine hydrochloride was 82.5 grams, or 37% of theory.
You might also like
- KINGS CHEMISTRY SURVIVAL GUIDE A Guide For The Hobbyist Enthusiast or Amateur For The Preparation of Common and Un Common Laboratory ChemicalsDocument134 pagesKINGS CHEMISTRY SURVIVAL GUIDE A Guide For The Hobbyist Enthusiast or Amateur For The Preparation of Common and Un Common Laboratory Chemicalszlobiul92% (38)
- Dimethylamine PDFDocument2 pagesDimethylamine PDFangelofgloryNo ratings yet
- Biosynthesis of EphedrineDocument12 pagesBiosynthesis of EphedrineGenceNo ratings yet
- Reductive Amination of Carbonyl Compounds With Borohydride and BoranDocument170 pagesReductive Amination of Carbonyl Compounds With Borohydride and BoranbhattavenuNo ratings yet
- A Convenient Way To Synthesis of Analgesic TramadolDocument1 pageA Convenient Way To Synthesis of Analgesic TramadolFacundo BaróNo ratings yet
- SYNTHETIC HOMOLOGS RESEARCHDocument6 pagesSYNTHETIC HOMOLOGS RESEARCHd4rk3No ratings yet
- Phenylacetic Acid From Benzyl CyanideDocument3 pagesPhenylacetic Acid From Benzyl CyanideriskobinskoNo ratings yet
- MeNH2 SynthesisDocument5 pagesMeNH2 Synthesisjiskate77No ratings yet
- Leuckart ReactionDocument3 pagesLeuckart ReactionKybernetikum100% (1)
- Amp 2 DMADocument5 pagesAmp 2 DMARenæ NaeNo ratings yet
- Phenylalanine +TCCA - Thread From WDDocument17 pagesPhenylalanine +TCCA - Thread From WDigremli100% (1)
- 20 Organic Chemistry Synthesis Iedxcel PDFDocument10 pages20 Organic Chemistry Synthesis Iedxcel PDFMohammedNo ratings yet
- Effects of Bath Salts Drug MDPVDocument21 pagesEffects of Bath Salts Drug MDPVOmar ZourobNo ratings yet
- Synthesis of Nitroalkanes From Bromoalkanes by Phase-Thansfer CatalysisDocument3 pagesSynthesis of Nitroalkanes From Bromoalkanes by Phase-Thansfer Catalysisscribd3822No ratings yet
- Arclightshroom's 4 Aco DMT WorkDocument6 pagesArclightshroom's 4 Aco DMT Workj.pedrogomes84No ratings yet
- KINGS CHEMISTRY SURVIVAL GUIDE A Guide For The Hobbyist Enthusiast or Amateur For The Preparation of Common and Un Common Laboratory Chemical PDFDocument134 pagesKINGS CHEMISTRY SURVIVAL GUIDE A Guide For The Hobbyist Enthusiast or Amateur For The Preparation of Common and Un Common Laboratory Chemical PDFvasiliyNo ratings yet
- 2,5-Dimethoxybenzaldehyde From 4-Methoxy PhenolDocument7 pages2,5-Dimethoxybenzaldehyde From 4-Methoxy PhenolSignora SauerNo ratings yet
- Purification of Organic Compounds by Re Crystallization MethodDocument4 pagesPurification of Organic Compounds by Re Crystallization Methodeljockey100% (2)
- MethDocument2 pagesMethhochaus123No ratings yet
- Procedures For The Resolution of Racemic AmphetaminesDocument4 pagesProcedures For The Resolution of Racemic AmphetaminesЯн ДенисенкоNo ratings yet
- Illicit Drug Labs Pose Serious Health RisksDocument9 pagesIllicit Drug Labs Pose Serious Health RisksAlexander Melo AnguloNo ratings yet
- Methods for Oxidation of Organic Compounds V2: Alcohols, Alcohol Derivatives, Alky Halides, Nitroalkanes, Alkyl Azides, Carbonyl Compounds Hydroxyarenes and AminoarenesFrom EverandMethods for Oxidation of Organic Compounds V2: Alcohols, Alcohol Derivatives, Alky Halides, Nitroalkanes, Alkyl Azides, Carbonyl Compounds Hydroxyarenes and AminoarenesNo ratings yet
- KsynDocument3 pagesKsynFlorianLiestNo ratings yet
- (R) - Phenylacetylcarbinol Production in Aqueous:organic Two-Phase Systems Using Partially Purified Pyruvate Decarboxylase From Candida UtilisDocument9 pages(R) - Phenylacetylcarbinol Production in Aqueous:organic Two-Phase Systems Using Partially Purified Pyruvate Decarboxylase From Candida UtilisMike RohrichNo ratings yet
- Total Synthesis II How To Make Ecstacy by StrikeDocument147 pagesTotal Synthesis II How To Make Ecstacy by StrikeRobert PeterssonNo ratings yet
- Benzaldehyde and Mek PatentDocument3 pagesBenzaldehyde and Mek Patentson100% (1)
- Preparation of MDMA by Reductive Amination With Sodium Borohydri PDFDocument5 pagesPreparation of MDMA by Reductive Amination With Sodium Borohydri PDFAshkan AbbasiNo ratings yet
- Organic Chem Lab FDocument33 pagesOrganic Chem Lab FRanjith Kumar mNo ratings yet
- Methylating Amphetamines SafelyDocument15 pagesMethylating Amphetamines Safelylocolocolocoxoxo100% (1)
- Improved Bromination of 2C HDocument3 pagesImproved Bromination of 2C HhappylmNo ratings yet
- Alexandra Doddridge, Michael Collins and Helen SalourosDocument26 pagesAlexandra Doddridge, Michael Collins and Helen SalourosDoc MartenzNo ratings yet
- Summation of The Sciencemadness Phosphorous ThreadDocument29 pagesSummation of The Sciencemadness Phosphorous ThreadImranNo ratings yet
- Synthesis of Acetophenone DerivativesDocument6 pagesSynthesis of Acetophenone DerivativesAwad SaidNo ratings yet
- Aldol Condensation Product IdentificationDocument5 pagesAldol Condensation Product IdentificationKatherine McLarneyNo ratings yet
- 7.3 (B) Preparing Standard SolutionDocument18 pages7.3 (B) Preparing Standard SolutionNovah GurulooNo ratings yet
- P2NP-derivation To The Alternative ApproachDocument3 pagesP2NP-derivation To The Alternative ApproachMoritz KaupNo ratings yet
- Nitro Alkene DerivativesDocument7 pagesNitro Alkene Derivativesgeovani2No ratings yet
- Metal—Ammonia Solutions: Proceedings of an International Conference on the Nature of Metal-Ammonia Solutions: Colloque Weyl IIFrom EverandMetal—Ammonia Solutions: Proceedings of an International Conference on the Nature of Metal-Ammonia Solutions: Colloque Weyl IINo ratings yet
- Organic Reactions v1Document396 pagesOrganic Reactions v1rhozab100% (5)
- Separation and Purification of Organic CompoundsDocument3 pagesSeparation and Purification of Organic CompoundsDonutHoshimiNo ratings yet
- 2-Methylamine Synthesis PictorialDocument9 pages2-Methylamine Synthesis Pictorialjiskate770% (1)
- Multicomponent Reactions via ortho-Quinone MethidesDocument74 pagesMulticomponent Reactions via ortho-Quinone MethidesRohan Prajapati100% (1)
- Os Coll. Vol. 6 P175-PtabDocument5 pagesOs Coll. Vol. 6 P175-Ptabsunil_vaman_joshiNo ratings yet
- Reteta p2pDocument2 pagesReteta p2pJohn JohnNo ratings yet
- Leuckart ReactionDocument4 pagesLeuckart ReactionangelofgloryNo ratings yet
- Amphetamine ReductionDocument3 pagesAmphetamine Reductiongardner88No ratings yet
- One Pot SynthesisDocument7 pagesOne Pot SynthesisvirparaNo ratings yet
- Eschweiler-Clarke Solventfree PDFDocument10 pagesEschweiler-Clarke Solventfree PDFRenæ NaeNo ratings yet
- Characterization of Three Methcathinone AnalogsDocument8 pagesCharacterization of Three Methcathinone AnalogsdoubleffectNo ratings yet
- MethDocument3 pagesMethAnonymous YviGuCNo ratings yet
- JCLIC July 2017Document58 pagesJCLIC July 2017Tj0% (1)
- Synthesis of P2P From 3-Phenyl-1-ChloropropaneDocument1 pageSynthesis of P2P From 3-Phenyl-1-ChloropropaneFlorianLiest100% (1)
- Bouveault-Blanc Ester ReductionDocument3 pagesBouveault-Blanc Ester ReductionAriel GarciaNo ratings yet
- Flaming Snowball Instruction & QuestionsDocument7 pagesFlaming Snowball Instruction & QuestionsJohn CenaNo ratings yet
- Methamphetamine: History, Cooking Methods, and DecontaminationDocument52 pagesMethamphetamine: History, Cooking Methods, and DecontaminationMarcus RamosNo ratings yet
- Birch Reduction Mechanism Explained in 40 CharactersDocument4 pagesBirch Reduction Mechanism Explained in 40 CharactersPawan BabelNo ratings yet
- Precautions:: Hydrofluoric AcidDocument2 pagesPrecautions:: Hydrofluoric AcidPrem KumarNo ratings yet
- Mastro Buon IDocument58 pagesMastro Buon IMagikFungusNo ratings yet
- Solvent Free Reduction of Aromatic Nitro Compounds With Alumina Supported Iron Powder and Acetic Acid Under Microwave IrradiationDocument5 pagesSolvent Free Reduction of Aromatic Nitro Compounds With Alumina Supported Iron Powder and Acetic Acid Under Microwave IrradiationKybernetikumNo ratings yet
- A-Bromination Using HBR H2O2 APKDocument7 pagesA-Bromination Using HBR H2O2 APKAshutosh BhaveNo ratings yet
- Alexandra Doddridge, Michael Collins and Helen SalourosDocument26 pagesAlexandra Doddridge, Michael Collins and Helen SalourosDoc MartenzNo ratings yet
- R12ii - A Review of Impurity Profiling and Synthetic RouteDocument19 pagesR12ii - A Review of Impurity Profiling and Synthetic Routedmar5No ratings yet
- Autocatalytic Oxidation of Ethers With Sodium BromateDocument6 pagesAutocatalytic Oxidation of Ethers With Sodium Bromatebebabebic45No ratings yet
- Please Wait... : View Cart & Checkout View Cart & CheckoutDocument14 pagesPlease Wait... : View Cart & Checkout View Cart & Checkoutryan jaridNo ratings yet
- Cocaine For TropinoneDocument8 pagesCocaine For TropinoneJi ChemNo ratings yet
- Criminal Code Regulation 2005Document44 pagesCriminal Code Regulation 2005IxariRomanNo ratings yet
- An Efficient Method For The Synthesis of 1,5-Benzodiazepine Derivatives Under Microwave Irradiation Without SolventDocument4 pagesAn Efficient Method For The Synthesis of 1,5-Benzodiazepine Derivatives Under Microwave Irradiation Without SolventHaouassi LakhdarNo ratings yet
- Determination of Safrole Content of Essential OilsDocument2 pagesDetermination of Safrole Content of Essential OilsUmakanthan KanagaratnamNo ratings yet
- Newer Methods of Preparative Organic Chemistry V2From EverandNewer Methods of Preparative Organic Chemistry V2Wilhelm FoerstNo ratings yet
- BioRes 05-3-1554 Zamani T Prod Low MW Chitosan Sulfuric 990Document11 pagesBioRes 05-3-1554 Zamani T Prod Low MW Chitosan Sulfuric 990riskobinskoNo ratings yet
- Zytiga Doctor Discussion Guide PDFDocument30 pagesZytiga Doctor Discussion Guide PDFriskobinskoNo ratings yet
- Spot Test For ReagentsDocument37 pagesSpot Test For ReagentsriskobinskoNo ratings yet
- Synthesis of Paredrine and Related CompoundsDocument5 pagesSynthesis of Paredrine and Related Compoundscmyk69No ratings yet
- Ritter Reaction SafroleDocument3 pagesRitter Reaction SafroleriskobinskoNo ratings yet
- Hex Am Ethylene Tetra MineDocument17 pagesHex Am Ethylene Tetra MineriskobinskoNo ratings yet
- 2012 June ISA CHEM6TP Question PaperDocument9 pages2012 June ISA CHEM6TP Question PaperjamesNo ratings yet
- Studies on Phosphorylation Part XIDocument4 pagesStudies on Phosphorylation Part XIEvan EsceNo ratings yet
- R. Minard - The Preparation of The Local Anesthetic, Benzocaine, by An Esterification ReactionDocument6 pagesR. Minard - The Preparation of The Local Anesthetic, Benzocaine, by An Esterification ReactionNstm3No ratings yet
- 2 BenzophenoneDocument3 pages2 BenzophenoneElizabeth LawsonNo ratings yet
- Cbse Xii Chemistry Project Preparation of Aspirin and AcetaminophenDocument7 pagesCbse Xii Chemistry Project Preparation of Aspirin and AcetaminophenVasudevSingh57% (7)
- Recrystallization of Acetanilide (2EMT - Group 1, 2009)Document7 pagesRecrystallization of Acetanilide (2EMT - Group 1, 2009)Mary Christelle100% (2)
- Bhopal Nobles Public School Chemistry Project FinalDocument11 pagesBhopal Nobles Public School Chemistry Project Finalbhawana vaishnavNo ratings yet
- Isolation of Piperine From Black PepperDocument8 pagesIsolation of Piperine From Black PepperRaadBassamNo ratings yet
- Chemistry Unit 3B NotesDocument13 pagesChemistry Unit 3B NotesShuchi HossainNo ratings yet
- STKK1032-Amali Kimia 1Document15 pagesSTKK1032-Amali Kimia 1Sharanya BhaskarNo ratings yet
- FFR 1Document9 pagesFFR 1Emily CribasNo ratings yet
- 2.2 Separation of Mixtures - 0Document25 pages2.2 Separation of Mixtures - 0Arch Chellis OrongNo ratings yet
- Potassium p-phenolsulfonate buffer and UV absorptionDocument8 pagesPotassium p-phenolsulfonate buffer and UV absorptionIinthand BEncii DyNo ratings yet
- A Novel and Green Route For Synthesis of Pyrazoline Derivatives in An Aqueous Media by Using Ionic Liquid at Reflux ConditionDocument4 pagesA Novel and Green Route For Synthesis of Pyrazoline Derivatives in An Aqueous Media by Using Ionic Liquid at Reflux ConditioniaetsdiaetsdNo ratings yet
- Aldol Puzzle Lab ReportDocument11 pagesAldol Puzzle Lab Reportlaurabruce27100% (1)
- Section-B: A Practical Book of Medicinal Chemistry-I (Sem. - V)Document19 pagesSection-B: A Practical Book of Medicinal Chemistry-I (Sem. - V)Raju NiraulaNo ratings yet
- Síntesis de 4,4-Diphenyl.... - V2Document4 pagesSíntesis de 4,4-Diphenyl.... - V2manuherreradariasNo ratings yet
- Anomalous Reaction of Epichlorohydrin With Trimethylamine by D. M. BurnessDocument3 pagesAnomalous Reaction of Epichlorohydrin With Trimethylamine by D. M. Burnessjohn_dominic_4No ratings yet
- Bromination and DebrominationDocument3 pagesBromination and DebrominationSmit PatelNo ratings yet
- Recrystallization and Melting Point Determination of Benzoic AcidDocument6 pagesRecrystallization and Melting Point Determination of Benzoic AcidAnonymous GO6JVW9Wud0% (1)
- EXercise 2 (Recrystallization and Melting Point Determination)Document3 pagesEXercise 2 (Recrystallization and Melting Point Determination)fangirltonNo ratings yet
- Organic Chemistry SIPCAnDocument15 pagesOrganic Chemistry SIPCAnAnthony NetzelNo ratings yet
- Heterogeneous Ditopic ZnFe2O4 Catalyzed Synthesis of 4h-Pyrans: Further Conversion To 1,4-DHPs and Report of Functional Group Interconversion From Amide To EsterDocument10 pagesHeterogeneous Ditopic ZnFe2O4 Catalyzed Synthesis of 4h-Pyrans: Further Conversion To 1,4-DHPs and Report of Functional Group Interconversion From Amide To EsterNGsalunkheNo ratings yet
- IAL Chemistry SB2 Answers Topic20Document5 pagesIAL Chemistry SB2 Answers Topic20salmaNo ratings yet