Professional Documents
Culture Documents
Cycloalkane Ring Strain and Conformations
Uploaded by
bgbscgv0 ratings0% found this document useful (0 votes)
322 views18 pages1. Cyclic hydrocarbons contain carbon atoms joined in a ring structure. Cycloalkanes have fully saturated carbon rings, while cycloalkenes contain double bonds in the ring.
2. The nomenclature for cyclic hydrocarbons involves prefixing the name with "cyclo-". No numbering is needed for functional groups in cyclic alkenes.
3. Smaller rings like cyclopropane and cyclobutane experience more angle, torsional, and steric strain than larger five- and six-membered rings. Cyclohexane is the most stable due to having bond angles and hydrogens in a staggered conformation that minimizes strain.
Original Description:
Original Title
Cyclic Alkanes & Alkenes
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. Cyclic hydrocarbons contain carbon atoms joined in a ring structure. Cycloalkanes have fully saturated carbon rings, while cycloalkenes contain double bonds in the ring.
2. The nomenclature for cyclic hydrocarbons involves prefixing the name with "cyclo-". No numbering is needed for functional groups in cyclic alkenes.
3. Smaller rings like cyclopropane and cyclobutane experience more angle, torsional, and steric strain than larger five- and six-membered rings. Cyclohexane is the most stable due to having bond angles and hydrogens in a staggered conformation that minimizes strain.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
322 views18 pagesCycloalkane Ring Strain and Conformations
Uploaded by
bgbscgv1. Cyclic hydrocarbons contain carbon atoms joined in a ring structure. Cycloalkanes have fully saturated carbon rings, while cycloalkenes contain double bonds in the ring.
2. The nomenclature for cyclic hydrocarbons involves prefixing the name with "cyclo-". No numbering is needed for functional groups in cyclic alkenes.
3. Smaller rings like cyclopropane and cyclobutane experience more angle, torsional, and steric strain than larger five- and six-membered rings. Cyclohexane is the most stable due to having bond angles and hydrogens in a staggered conformation that minimizes strain.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 18
1
1.5 Cyclic Alkanes / Alkenes
1.5.1 Structure
A hydrocarbon that contains carbon atoms joined to
form a ring is called a cyclic hydrocarbon.
When all carbons of the ring are saturated (sp
3
), the
hydrocarbon is called cycloalkane.
When a double bond (sp
2
), is part of the ring, the hydrocarbon
is called cycloalkene.
2
1.5.2 Nomenclature
The system for naming members of this class is
straightforward. Alkane/Alkene names are preceded by the
prefix cyclo-
However, no numbering of the functional group is
needed in a cyclic alkene
Examples
3
4
Some definitions:
Angle strain: it is the strain induced in a molecule when the
bond angles are different from the ideal tetrahedral bond
angle of 109.5o.
Torsional strain: it is caused by repulsion between the
bonding electrons of one substituent and the bonding
electrons of a nearby substituent.
Steric strain: it is caused by atoms or groups of atoms
approaching each other too closely.
Total Strain Energies of Selected Cycloalkanes
0 Cyclohexane
25.9 Cyclopentane
110.9 Cyclobutane
114.2 Cyclopropane
Strain Energy (kJ/mol) Alkane
1.5.3 Ring Strain and the Structure of Cycloalkanes
5
Generally speaking, cyclic alkanes found in nature have five
or six-membered rings. On the other hand, compounds with
three and four-membered rings are found much less
frequently. This observation suggested that alkanes with
five- and six-membered rings must be more stable than
those with three- or four-membered rings. It was proposed
that such instability could be explained on the bases of
angle strain. Ideally, an sp
3
hybridized carbon has bond
angles of 109.5. As a result, stability of a cycloalkane may
be predicted by determining how close the bond angle of a
planar cycloalkane is to 109.5
0
. The angles of an equilateral
triangle are 60
o
. Therefore, the bond angles in a planar
cyclopropane are compressed from the ideal bond angle
of 109.5
o
to 60
o
, a 49.5
o
deviation causing angle strain.
6
As described earlier, normal sigma bond between two carbon
atoms are formed by the overlap of two sp
3
orbitals that
point directly at each other. In cyclopropane, overlapping
orbitals cannot point directly at each other.
Therefore, the orbital overlap is less effective than in a
normal C-C bond. Hence, the less effective orbital overlap
causes the C-C bond to be weaker and could be easily
broken i.e. reactive. For example, cyclopropane could be
readily hydrogenated to propane.
H
2
Cyclopropane
Propane
Pt
7
Because the C-C bonding orbitals in Cyclopropane cannot
point directly at each other, they have shapes that resemble
bananas and, consequently, are often called banana bonds.
In addition to angle strain, three-membered rings have
torsional strain as a result of the fact that all hydrogen atoms
are eclipsed.
8
Similarly, the bond angles in planar cyclobutane would
have to be compressed from 109.5
o
to 90
o
, the bond angle
associated with a planar square. Planar cyclobutane would
then be expected to have less angle strain than
cyclopropane because the bond angles in cyclobutane are
only 19.5
o
away from the ideal angle.
Considering angle strain as the only factor, it was predicted
that cyclopentane be the most stable of cycloalkanes
because its bond angles (108
o
) are closest to the ideal
tetrahedral one.
In addition, it may be predicted that cyclohexane, with bond
angles of 120
o
, would be less stable.
9
Because three points define a plane, the carbons of
cyclopropane indeed lie in a plane as it cannot twist.
As a result cyclpropane is planar.
On the other hand, other cycloalkanes are not planar. They
are capable of twisting and bend in order to attain a
structure that minimizes the three different kind of strain
(angle, torsional, and steric strains) that destabilize a cyclic
compound.
Contrary to this prediction, it turned out that cyclohexane is
more stable than the five-membered ring! Why?
The assumption that all cyclic molecules are planar is not
accurate.
10
Although planar cyclobutane would have less angle strain than
cyclopropane, it could have more torsional strain because it has eight
pairs of eclipsed hydrogens, compared with the six of cyclopropane.
Hence, cyclobutane is not planar molecule-it is bent molecule. Although
this increases the angle strain never the less, the increase is
more than compensated for by the decreased torsional strain.
110.9
Cyclobutane
114.2 Cyclopropane
Strain Energy (kJ/mol) Alkane
Similarly, if cyclopentane were planar, it would have essentially no angle
strain. In this case however, its 10 pairs of eclipsed hydrogens would be
subject to considerable torsional strain. Consequently, cyclopentane
puckers, allowing the hydrogens to become nearly staggered
although in doing so it acquires some angle strain.
0 Cyclohexane
25.9 Cyclopentane
Strain Energy
(kJ/mol)
Alkane
11
Such form is called the envelope conformation as the shape resembles
an envelope with the flap up.
In contrast to smaller rings, distortion from planarity in cyclohexane
relieves both the angle and torsional strain of the planar structure.
Once more, the internal angle in a planar hexagon is 120
o
, larger, not
smaller, than the ideal sp
3
angle. Deviation from planarity will decrease
both this angle and torsional strain from the six pairs of eclipsed
hydrogens in planar model.
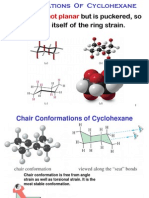
Remarkably, this relaxation produces a molecule in which
essentially all of the torsional and angle strain is gone. This
energy minimum cyclohexane is called the chair form. In the
chair conformer of cyclohexane, all bond angles are 111
o
and all the adjacent bonds are staggered.
12
Definitions
Equatorial carbon-hydrogen bonds are parallel to the ring
carbon-carbon bonds one bond away in chair cyclohexane.
Axial carbon-hydrogen bonds are parallel and pointing either
straight up or down in chair cyclohexane
13
Cyclohexane rapidly interconverts between two stable chair conformations
because of the ease of rotation about its C-C bonds. Such process is called ring
flip. When the chair conformers interconvert, bonds that equatorial in one chair
conformer become axial in the other chair conformer and vice versa.
Definitions: Equatorial carbon-hydrogen bonds are parallel to the ring carbon-
carbon bonds one bond away in chair cyclohexane. Axial carbon-hydrogen bonds
are parallel and pointing either straight up or down in chair cyclohexane
14
Cyclohexane can also exist in a boat conformation
Similar to the chair conformer, the boat conformer is free of
angle strain. However, the boat conformer is not as stable
because some of its bonds are eclipsed, giving torsional
strain to the molecule. In addition, the boat conformer is
further destabilized by the close proximity of the
flagpole hydrogens which causes steric strain.
15
16
H
H
H
H
H
H
H
H
CH2
CH2
Newman projection of the chair conformer
In the boat form, the bonded atoms are in
the less stable eclipsed conformation,
whereas in the chair form, they are staggered
1
4
1
4
In the boat form, carbons 1, 4
Are pulled toward each other,
Causing steric interactions between
The flagpole hydrogens.
In the chair form, these same carbons are
bent away from each other, and thus are
not subject to mutual repulsion.
17
It should be noted that while cyclohexane interconverts from one chair
conformer to the other, it can assume other conformations namely, half-chair
and twist-boat. As expected, because the chair conformers are the most stable
conformers, at any instant more molecules of cyclohexane are in chair
conformations than in any other one.
Interesting to note that it has been calculated that, for every thousand molecules
of cyclohexane in a chair conformation, no more than two molecules are in the
next most stable conformations-the twist-boat. Cis trans in cycloalkanes
18
1.6 Functional groups
1.6.1 Definition
It is the part of a molecule where most of its chemical
reactions occur. Alkanes do not have a functional group. It
is the part that effectively determines the compounds
chemical and most of its physical properties.
1.6.2 Most common functional groups
Aldehyde Ketone Carboxylic acidCarboxylic ester Alcohol
Ether Alkene Alkyne
R C
H
H
OH
R C
H
O
C O
R
R
C
O
OH R
R O R
R C O R
O
R NH
2
R C NH
2
O
Amine
Amide
R C C R
C
H
H
C
R
R
You might also like
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- Angle Strain Torsional Strain Ring StrainDocument6 pagesAngle Strain Torsional Strain Ring StrainchuasioklengNo ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- Cyclic Aliphatic Compounds: NomenclatureDocument19 pagesCyclic Aliphatic Compounds: NomenclatureWinnie SantiagoNo ratings yet
- Module 5: Conformational analysis of alkanes and cyclohexanesDocument8 pagesModule 5: Conformational analysis of alkanes and cyclohexanesARMAN AKRAM BIN OMAR / UPMNo ratings yet
- Cycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheoryDocument5 pagesCycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheorySayed AltamashNo ratings yet
- Conformation & Conformational IsomersDocument4 pagesConformation & Conformational Isomerspulkit asatiNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- Cyclohexane Conformational AnalysisDocument1 pageCyclohexane Conformational Analysis휘승No ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Tutorial07b OrganicCycloalkanesDocument24 pagesTutorial07b OrganicCycloalkanesHana NisrinaNo ratings yet
- Coulson and MoffittDocument2 pagesCoulson and MoffittTanya 60No ratings yet
- Lesson StructureDocument19 pagesLesson Structurer karthickNo ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Conformational Analysis: Understanding How Molecule Shapes Impact PropertiesDocument10 pagesConformational Analysis: Understanding How Molecule Shapes Impact PropertiesPG Chemistry PG ChemistryNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- 4 Cyclohexane: Axial Bond Equatorial BondDocument13 pages4 Cyclohexane: Axial Bond Equatorial BondAminahNatashaNo ratings yet
- SCH 102 Lecture Week 8 2018-9Document27 pagesSCH 102 Lecture Week 8 2018-9Radhika TopwalNo ratings yet
- Cycloalkanes are Nonplanar Due to Strain FactorsDocument29 pagesCycloalkanes are Nonplanar Due to Strain FactorsjuanNo ratings yet
- Org Chem Text - Chapter 1 - Sec1-14 - 1-14Document3 pagesOrg Chem Text - Chapter 1 - Sec1-14 - 1-14TET2005No ratings yet
- BSES Topic 06 Cycloalkanes and Aromatic CompoundsDocument42 pagesBSES Topic 06 Cycloalkanes and Aromatic CompoundsJhunessa Mae JalagatNo ratings yet
- Org Lec 2Document27 pagesOrg Lec 2rupayandaripaNo ratings yet
- Cyclo AlkanesDocument38 pagesCyclo Alkanesdinesh111180No ratings yet
- InorganicDocument43 pagesInorganicShobhit ZakhmiNo ratings yet
- Introduction to AlkanesDocument26 pagesIntroduction to AlkanesShinta Novita SariNo ratings yet
- Conformational IsomersDocument8 pagesConformational Isomersshirodkar_16593No ratings yet
- Monocyclic DisubstitutedDocument3 pagesMonocyclic DisubstitutedPs SatchithNo ratings yet
- Chapter 3: Structure and Stereochemistry of AlkanesDocument42 pagesChapter 3: Structure and Stereochemistry of AlkanesKera Maria AllengerNo ratings yet
- UntitledDocument42 pagesUntitledRAVI5268No ratings yet
- Alkanes, Alkenes, Alkynes, and CycloalkanesDocument162 pagesAlkanes, Alkenes, Alkynes, and CycloalkanesNurtasha AtikahNo ratings yet
- Unit_5 (1)Document42 pagesUnit_5 (1)RAVI5268No ratings yet
- DecalinsDocument25 pagesDecalinstessyNo ratings yet
- Organic Chemistry: M. R. Naimi-JamalDocument81 pagesOrganic Chemistry: M. R. Naimi-JamalHamidatun NisaNo ratings yet
- What Are Cyclo-Alkanes?Document6 pagesWhat Are Cyclo-Alkanes?gfgfccNo ratings yet
- Cyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Document6 pagesCyclo-Alkanes: Pharmaceutical Organic Chemistry - Ii (Theory)Tushar GiradkarNo ratings yet
- Cyclohexane Conformational AnalysisDocument17 pagesCyclohexane Conformational AnalysiscclatrumNo ratings yet
- Conformational Isomerism in AlkanesDocument15 pagesConformational Isomerism in AlkanesNitya BhartiNo ratings yet
- Conformations and Strains of Alkanes and CycloalkanesDocument92 pagesConformations and Strains of Alkanes and CycloalkanesMutia HadiyantiNo ratings yet
- ISOMERIA: Conformaciones y configuraciones diferentesDocument45 pagesISOMERIA: Conformaciones y configuraciones diferentesJuan CarlosNo ratings yet
- CycloalkanesDocument18 pagesCycloalkanesJohn Mark PolisticoNo ratings yet
- Relative Stabilities of CycloakanesDocument8 pagesRelative Stabilities of CycloakanesKamal KishoreNo ratings yet
- CONFORMATIONSDocument3 pagesCONFORMATIONSElaaf AnzarNo ratings yet
- Conformations IIDocument16 pagesConformations IIsndNo ratings yet
- Cyclic AlkanesDocument35 pagesCyclic AlkanesKunjal100% (2)
- 4 CH241 CycloalkanesDocument73 pages4 CH241 CycloalkanesLuis CastilloNo ratings yet
- Organic Lecture 2Document27 pagesOrganic Lecture 2Dhruv RaiNo ratings yet
- Molecular Shapes and Hybridisation TheoryDocument37 pagesMolecular Shapes and Hybridisation TheoryChai Kah ChunNo ratings yet
- Organic - Class 8Document41 pagesOrganic - Class 8Sajan Singh LUCKYNo ratings yet
- Baeyer's Strain Theory: Postulates and AssumptionsDocument13 pagesBaeyer's Strain Theory: Postulates and AssumptionsSomali SenguptaNo ratings yet
- Cyclo Al KanesDocument3 pagesCyclo Al KanesIbraim ZekirNo ratings yet
- Tutorial 15Document5 pagesTutorial 15Pace AjjaNo ratings yet
- CHEM F111: General Chemistry Lecture on Conformational Isomers and CycloalkanesDocument17 pagesCHEM F111: General Chemistry Lecture on Conformational Isomers and CycloalkanesRachit ShahNo ratings yet
- 1515564149CHE P1 M16 EtextDocument22 pages1515564149CHE P1 M16 EtextElangovan NatarajanNo ratings yet
- LecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryDocument8 pagesLecturerNotes BPharmIIISem PCISyllabus UNIT V CycloalkanesStrainTheoryKevalNo ratings yet
- Conformational Analysis:: Chapter - 6 Stereochemistry NotesDocument12 pagesConformational Analysis:: Chapter - 6 Stereochemistry NotesMohit KambojNo ratings yet
- Organic Compounds:: Cycloalkanes and Their StereochemistryDocument34 pagesOrganic Compounds:: Cycloalkanes and Their StereochemistryrilaNo ratings yet
- Chair & BoatDocument9 pagesChair & BoatpardeepbthNo ratings yet
- Conformation AnalysisDocument41 pagesConformation AnalysisJoseNo ratings yet
- A New System of Alternating Current Motors and TransformersFrom EverandA New System of Alternating Current Motors and TransformersRating: 1 out of 5 stars1/5 (1)
- BlockStack Top 21 Trends in Web3 1676290935Document9 pagesBlockStack Top 21 Trends in Web3 1676290935Ahmed BachaNo ratings yet
- Practical Exercise - Analysis of The Collapse of Silicon Valley BankDocument2 pagesPractical Exercise - Analysis of The Collapse of Silicon Valley Bankhanna.ericssonkleinNo ratings yet
- A1 Paper4 TangDocument22 pagesA1 Paper4 Tangkelly2999123No ratings yet
- Abcdef Ghijkl Mnopq Rstuv Wxyz Alphabet Backpack Book Bookcase CalculatorDocument4 pagesAbcdef Ghijkl Mnopq Rstuv Wxyz Alphabet Backpack Book Bookcase Calculatornutka88No ratings yet
- Essay 'Why Alice Remain Popular?'Document3 pagesEssay 'Why Alice Remain Popular?'Syamil AdzmanNo ratings yet
- The Reaction Between Potassium Permanganate and Oxalz'c AcidDocument3 pagesThe Reaction Between Potassium Permanganate and Oxalz'c AcidNorazwan NorNo ratings yet
- Family Values, Livelihood Resources and PracticesDocument285 pagesFamily Values, Livelihood Resources and PracticesRogelio LadieroNo ratings yet
- MNL036Document22 pagesMNL036husni1031No ratings yet
- Activity-Sheet-Module 1 Relation and FunctionDocument7 pagesActivity-Sheet-Module 1 Relation and FunctionNeah Neoh NeohnNo ratings yet
- The Retired Adventurer - Six Cultures of PlayDocument14 pagesThe Retired Adventurer - Six Cultures of Playfernando_jesus_58No ratings yet
- Interventional Radiology & AngiographyDocument45 pagesInterventional Radiology & AngiographyRyBone95No ratings yet
- Anxiety Free - Stop Worrying and - McKeown, PatrickDocument237 pagesAnxiety Free - Stop Worrying and - McKeown, PatrickLoboCamon100% (1)
- Mutual FundDocument40 pagesMutual Fundn kNo ratings yet
- Osmaan Shamsiddeen: Work History Personal InfoDocument1 pageOsmaan Shamsiddeen: Work History Personal InfoOsmaan ShamsiddeenNo ratings yet
- An Adaptive Memoryless Tag Anti-Collision Protocol For RFID NetworksDocument3 pagesAn Adaptive Memoryless Tag Anti-Collision Protocol For RFID Networkskinano123No ratings yet
- Work of Juan TamarizDocument6 pagesWork of Juan Tamarizmrbookman3No ratings yet
- Name CompilationDocument490 pagesName CompilationMark Taylor100% (1)
- Management of Chronic ITP: TPO-R Agonist Vs ImmunosuppressantDocument29 pagesManagement of Chronic ITP: TPO-R Agonist Vs ImmunosuppressantNiken AmritaNo ratings yet
- 20 Đề Thi Thử Tiếng Anh Có Đáp Án Chi Tiết - LovebookDocument462 pages20 Đề Thi Thử Tiếng Anh Có Đáp Án Chi Tiết - LovebookPhương UyênNo ratings yet
- GUIDE FOR ROOM EXAMINER - 2023-05 Revised (CSE-Pen & Paper Test) - ModifiedDocument14 pagesGUIDE FOR ROOM EXAMINER - 2023-05 Revised (CSE-Pen & Paper Test) - ModifiedLeilani BacayNo ratings yet
- Chapter 2Document20 pagesChapter 2Saman Brookhim100% (4)
- Deflected Profile of A BeamDocument2 pagesDeflected Profile of A BeamPasindu MalithNo ratings yet
- Retool Your Linux Skills For Commercial UNIXDocument19 pagesRetool Your Linux Skills For Commercial UNIXPauloNo ratings yet
- Crema Coffee Garage - Understanding Caffeine Content of Popular Brewing Methods Within The Australian Coffee Consumer MarketDocument33 pagesCrema Coffee Garage - Understanding Caffeine Content of Popular Brewing Methods Within The Australian Coffee Consumer MarketTDLemonNhNo ratings yet
- Aits 2223 PT III Jeea Paper 1Document15 pagesAits 2223 PT III Jeea Paper 1Suvrajyoti TaraphdarNo ratings yet
- Chennai Contact - 1Document12 pagesChennai Contact - 1Jvr SubramaniaraajaaNo ratings yet
- Consent To Electronic CommunicationsDocument2 pagesConsent To Electronic CommunicationsVilmarie RiveraNo ratings yet
- Sales & Distribution Management Presentation NewDocument35 pagesSales & Distribution Management Presentation NewVivek Sinha0% (1)
- Classroom Management StrategiesDocument19 pagesClassroom Management Strategiesalmors0% (1)
- Pavement Design and Maintenance: Asset Management Guidance For Footways and Cycle RoutesDocument60 pagesPavement Design and Maintenance: Asset Management Guidance For Footways and Cycle RoutesGaneshmohiteNo ratings yet