Professional Documents
Culture Documents
C1-C3 Revision Mat
Uploaded by
Mrs S BakerCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
C1-C3 Revision Mat
Uploaded by
Mrs S BakerCopyright:
Available Formats
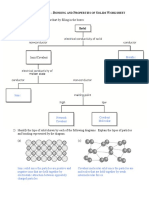
Draw a structure of the atom S3
How are electrons arranged in S4 Write the definitions of S2
and label the sub-atomic particles.
an atom. Element
Compound
Mixture
Describe the properties of proton, neutron and S4
electron.
Write the names of group 1, group 7 S8 Atomic Sub-atomic Mass Charge
Structure
and group 0 elements. particle
Proton
Neutron
What is the definition of an isotope? S5
Electron
Give two examples
Describe the reactivity S7,
Of alkali metals and 10
halogens.
Alkali metals
Explain why the reactivity increases S9
Halogen Explain why the reactivity increases S10
In alkali metals
in halogens.
Name the three different kinds S2 Draw an ionic bond for sodium and chlorine S3 Give two example of ionic bonds S3
of Bonding 1.
2.
State three properties of ionic bonds S4
How is a metallic bond formed S8 C2 – Structure
and Bonding Draw a diagram of covalent S5
bond in chlorine
Draw a diagram of S8
metallic bonds. Name the reaction of how polymers S9
are made
How is a covalent bond formed? S5
State four properties of
S6
Simple covalent bonds
Define relative S3
Formula mass.
Describe how polymers are made. S9 State four properties of giant covalent S7
structures?
You might also like
- SECOND GRADING PERIOD UNIT ON MATTER AND CHEMICAL BONDSDocument4 pagesSECOND GRADING PERIOD UNIT ON MATTER AND CHEMICAL BONDSShellane Blanco SarduaNo ratings yet
- Yr 12 Module 2 Electrons and OrbitalsDocument48 pagesYr 12 Module 2 Electrons and OrbitalsVikki McIntoshNo ratings yet
- Properties of Matter: Covalent Bonds, Molecular Geometry & PolarityDocument27 pagesProperties of Matter: Covalent Bonds, Molecular Geometry & PolarityJannaNo ratings yet
- When Atoms Meet: Chemical BondingDocument88 pagesWhen Atoms Meet: Chemical BondingWilsonNo ratings yet
- Structural Analysis of NanomaterialsDocument27 pagesStructural Analysis of NanomaterialswinnieNo ratings yet
- Math Tutor: Drawing Lewis StructuresDocument7 pagesMath Tutor: Drawing Lewis Structuresjaber bentabetNo ratings yet
- Chemistry I ASC 0304: Chemical BondsDocument35 pagesChemistry I ASC 0304: Chemical BondshadassahhadidNo ratings yet
- Points To Study For The TestDocument2 pagesPoints To Study For The TestSarah LindemannNo ratings yet
- Chemical BondingDocument3 pagesChemical Bondingparth ladaniNo ratings yet
- Gen Chem Module 6 Answer PDFDocument5 pagesGen Chem Module 6 Answer PDFjhon paul espinarNo ratings yet
- Chapter 7 Ionic and Metallic BondingDocument56 pagesChapter 7 Ionic and Metallic BondingCharles GibbsNo ratings yet
- Structure of Solids 1Document5 pagesStructure of Solids 1zakNo ratings yet
- Lecture 1.1 Organic Chemistry - MKDocument59 pagesLecture 1.1 Organic Chemistry - MKqurrelNo ratings yet
- F321 Bonding and StructureDocument10 pagesF321 Bonding and StructureDoc_Croc100% (1)
- Unit 3 - Chemical BondingDocument58 pagesUnit 3 - Chemical BondingTrang Vũ Thị BằngNo ratings yet
- SGCH 09Document24 pagesSGCH 09belleblackNo ratings yet
- Models of Chemical Bonding: Dr. Nur Farhana Binti JaafarDocument50 pagesModels of Chemical Bonding: Dr. Nur Farhana Binti JaafarNur HananiNo ratings yet
- 313 Chemistry Eng Lesson4Document37 pages313 Chemistry Eng Lesson4jdinnovate23No ratings yet
- 4 Chemical Bonding - After Review - 8!10!2019Document24 pages4 Chemical Bonding - After Review - 8!10!2019AFAQ HYDNo ratings yet
- F&Q - Segundo Examen - Presentación de IdoyaDocument15 pagesF&Q - Segundo Examen - Presentación de IdoyaSamuel Echeverría MuroNo ratings yet
- Bonding Structures & PropertiesDocument23 pagesBonding Structures & PropertiesRuha VNo ratings yet
- Yr 12 Module 2 BondingDocument28 pagesYr 12 Module 2 BondingVikki McIntoshNo ratings yet
- C3 Student Checklist: Structure and BondingDocument4 pagesC3 Student Checklist: Structure and BondingAngi SNo ratings yet
- Structure and BondingDocument12 pagesStructure and BondingNisha JodhanNo ratings yet
- Types of Solids POGIL - Student VersionDocument6 pagesTypes of Solids POGIL - Student VersionJesse SchwartzNo ratings yet
- Group 1 - Valence and Electron ConfigurationDocument12 pagesGroup 1 - Valence and Electron ConfigurationJulio GonzalezNo ratings yet
- Metallic BondingDocument17 pagesMetallic Bondingaudrey.sengeNo ratings yet
- Chapter7 Chemical Bonding Molecular Structure STUDDocument35 pagesChapter7 Chemical Bonding Molecular Structure STUDCristian Menéndez FernándezNo ratings yet
- 1.7.5 Covalent Bond RevisionDocument3 pages1.7.5 Covalent Bond RevisionTomáš Tommy NagyNo ratings yet
- Molecular Orbital TheoryDocument58 pagesMolecular Orbital Theoryvatsala soniNo ratings yet
- Inorganic Chemistry Lesson 10 CHEMICAL BONDING PDFDocument41 pagesInorganic Chemistry Lesson 10 CHEMICAL BONDING PDFKayra Myke VelascoNo ratings yet
- Structures of SolidsDocument6 pagesStructures of SolidsAlex noslenNo ratings yet
- Introductory Chemistry: Chemical BondDocument42 pagesIntroductory Chemistry: Chemical BondmayNo ratings yet
- Chemistry Unit 2 Study Guide AnswersDocument6 pagesChemistry Unit 2 Study Guide AnswersH.sNo ratings yet
- Chemical Bonding WorksheetDocument2 pagesChemical Bonding WorksheetIsabel Del ValleNo ratings yet
- Bonding and Properties of Solids Worksheet Solutions 1kadax6Document4 pagesBonding and Properties of Solids Worksheet Solutions 1kadax6Mel Patricia M. CabreraNo ratings yet
- Topic 4 Bonding NotesDocument13 pagesTopic 4 Bonding NotesThaarvena RetinaNo ratings yet
- 3 Chemistry For Engineers Chemical BondsDocument32 pages3 Chemistry For Engineers Chemical BondsHanzly AurellanoNo ratings yet
- C2-The Structure of The Atom - 0001Document25 pagesC2-The Structure of The Atom - 0001Christina T Z-chYnNo ratings yet
- Ncert Solutions March9 For Class 11 Chemistry Chapter 4Document32 pagesNcert Solutions March9 For Class 11 Chemistry Chapter 4Sarojini MallickNo ratings yet
- Chemical Bonding - Ionic BondDocument56 pagesChemical Bonding - Ionic BondFianna GalleroNo ratings yet
- MODULE 2 Chemical BondsDocument9 pagesMODULE 2 Chemical BondsMeah Liezl EmbodoNo ratings yet
- AQA Combined Science Structure and BondingDocument2 pagesAQA Combined Science Structure and Bondingali.a.226No ratings yet
- Types of Solids: Properties and CategoriesDocument6 pagesTypes of Solids: Properties and Categoriesjoninna monesNo ratings yet
- Fbise Chapter 4 (I)Document7 pagesFbise Chapter 4 (I)Zarish ZubairNo ratings yet
- TR - Dominic s2Document112 pagesTR - Dominic s2hervemanzi498No ratings yet
- Chemical Bonding NCERT Highlighted PDF White - ExtractedDocument5 pagesChemical Bonding NCERT Highlighted PDF White - ExtractedAkshraNo ratings yet
- A2AS CHEM REVISED Support 20837Document6 pagesA2AS CHEM REVISED Support 20837Tianming KingsleyNo ratings yet
- Notes - Chemical BondingDocument14 pagesNotes - Chemical Bonding黄心盈No ratings yet
- CH 2 Chemical Bonding PDFDocument26 pagesCH 2 Chemical Bonding PDFTonald DrumpNo ratings yet
- CHEM Types of Solids POGILDocument7 pagesCHEM Types of Solids POGILKosakenNo ratings yet
- Covalent Network Compounds and Their PropertiesDocument19 pagesCovalent Network Compounds and Their PropertiesmuhajireenNo ratings yet
- BondingDocument32 pagesBondingRalph Rezin MooreNo ratings yet
- Structure and Bonding - Ionic + Covalent BondsDocument9 pagesStructure and Bonding - Ionic + Covalent BondsKimaya RichardsNo ratings yet
- Metals and Nonetals - 3 Ionic CompoundDocument1 pageMetals and Nonetals - 3 Ionic Compoundbhumika motiyaniNo ratings yet
- Crystals Unit Cell Lattice FCC BCCDocument12 pagesCrystals Unit Cell Lattice FCC BCCPatrick Joshua GregorioNo ratings yet
- WRITING THE CHEMICAL FORMULA OF COVALENT COMPOUNDS-form 4Document19 pagesWRITING THE CHEMICAL FORMULA OF COVALENT COMPOUNDS-form 4Shanice JohnsonNo ratings yet
- BondingDocument45 pagesBondingRalph Rezin MooreNo ratings yet
- Information For Candidates - Privacy NoticeDocument2 pagesInformation For Candidates - Privacy NoticeMrs S BakerNo ratings yet
- B2 Photosynthesis Revision: Key ConceptsDocument7 pagesB2 Photosynthesis Revision: Key ConceptsMrs S BakerNo ratings yet
- Information For Candidates - Social Media 1718 PDFDocument1 pageInformation For Candidates - Social Media 1718 PDFMrs S BakerNo ratings yet
- Information For Cands cswk.1819 PDFDocument2 pagesInformation For Cands cswk.1819 PDFMrs S BakerNo ratings yet
- B1 CellsDocument7 pagesB1 CellsMrs S BakerNo ratings yet
- B7 Variation and EvolutionDocument7 pagesB7 Variation and EvolutionMrs S BakerNo ratings yet
- Aqa 7401 7402 SP 2015Document75 pagesAqa 7401 7402 SP 2015api-422428700No ratings yet
- Information For Candidates - Written Exams PDFDocument1 pageInformation For Candidates - Written Exams PDFMrs S BakerNo ratings yet
- Syllabus 2015Document88 pagesSyllabus 2015api-377518221No ratings yet
- B1 CellsDocument7 pagesB1 CellsMrs S BakerNo ratings yet
- C3 C5revisionmatDocument3 pagesC3 C5revisionmatMrs S BakerNo ratings yet
- P7 Electromagnetism MindmapDocument1 pageP7 Electromagnetism MindmapMrs S BakerNo ratings yet
- AQA A Level Chemistry SpecificationDocument84 pagesAQA A Level Chemistry Specificationastargroup100% (1)
- B1 Cells-Paper1 Revision: Key ConceptsDocument7 pagesB1 Cells-Paper1 Revision: Key ConceptsMrs S BakerNo ratings yet
- B5 Coordination and Control RevisionDocument18 pagesB5 Coordination and Control RevisionMrs S BakerNo ratings yet
- Keeping Healthy: Key ConceptsDocument11 pagesKeeping Healthy: Key ConceptsMrs S Baker100% (1)
- c8 Revision MatDocument1 pagec8 Revision MatMrs S BakerNo ratings yet
- B3 Moving and Changing MaterialsDocument10 pagesB3 Moving and Changing MaterialsMrs S BakerNo ratings yet
- C10 RevisionDocument7 pagesC10 RevisionMrs S BakerNo ratings yet
- C6 Rate and Extent of Chemical ChangeDocument2 pagesC6 Rate and Extent of Chemical ChangeMrs S BakerNo ratings yet
- P5 Forces Mind MapDocument1 pageP5 Forces Mind MapMrs S BakerNo ratings yet
- P6 Waves Mind MapDocument1 pageP6 Waves Mind MapMrs S BakerNo ratings yet
- C9 C10revisionmatDocument2 pagesC9 C10revisionmatMrs S BakerNo ratings yet
- C9 RevisionDocument8 pagesC9 RevisionMrs S BakerNo ratings yet
- C 6 RevisionDocument5 pagesC 6 RevisionMrs S BakerNo ratings yet
- C8 RevisionDocument6 pagesC8 RevisionMrs S BakerNo ratings yet
- C8: Chemical Analysis: Key ConceptsDocument6 pagesC8: Chemical Analysis: Key ConceptsMrs S BakerNo ratings yet
- C7: Hydrocarbons: Key ConceptsDocument8 pagesC7: Hydrocarbons: Key ConceptsMrs S BakerNo ratings yet
- C8: Chemical Analysis: Key ConceptsDocument6 pagesC8: Chemical Analysis: Key ConceptsMrs S BakerNo ratings yet
- c6 RevisionDocument5 pagesc6 RevisionMrs S BakerNo ratings yet
- A510m 06Document7 pagesA510m 06psewag100% (1)
- DBMS Queries OverviewDocument98 pagesDBMS Queries OverviewAbhinay YadavNo ratings yet
- Application of Nitrous Oxide in AutomobilesDocument26 pagesApplication of Nitrous Oxide in AutomobilesMohammed NuhmanNo ratings yet
- SSC Questions On Ratio and Proportion PDFDocument7 pagesSSC Questions On Ratio and Proportion PDFRobert ShortNo ratings yet
- Solid angles in perspective: Ω, have a small but essential role in physics. For example, howDocument8 pagesSolid angles in perspective: Ω, have a small but essential role in physics. For example, howashkarkabeer08No ratings yet
- Speed Control of DC Shunt MotorDocument7 pagesSpeed Control of DC Shunt MotorAakash0% (1)
- Cocoa Fermentation ManualDocument18 pagesCocoa Fermentation ManualJimena Rios100% (1)
- The Relative Pricing of High-Yield Debt: The Case of RJR Nabisco Holdings Capital CorporationDocument24 pagesThe Relative Pricing of High-Yield Debt: The Case of RJR Nabisco Holdings Capital CorporationAhsen Ali Siddiqui100% (1)
- MDR PDFDocument34 pagesMDR PDFJohnNo ratings yet
- Pritchett Clock Repair Shop Breakeven Analysis ExcelDocument138 pagesPritchett Clock Repair Shop Breakeven Analysis ExcelMohd Yousuf MasoodNo ratings yet
- Data MiningDocument48 pagesData MiningJAYASREE K.KNo ratings yet
- Classification of The Concha Type Microtia and Their New Suitable Tratment Strategies Without Autogenous Costal Cartilage GraftingDocument7 pagesClassification of The Concha Type Microtia and Their New Suitable Tratment Strategies Without Autogenous Costal Cartilage Graftingromina paz morales camposNo ratings yet
- Danfoss PVE - Series - 7 - Technical - Information PDFDocument56 pagesDanfoss PVE - Series - 7 - Technical - Information PDFJuan Felipe Garza GNo ratings yet
- SAT-101 User Manual: Document No: MAN-0013 Issue No: 4 Dated: 24 Aug 2004Document24 pagesSAT-101 User Manual: Document No: MAN-0013 Issue No: 4 Dated: 24 Aug 2004VM ServicesNo ratings yet
- Two-, Three-, and Four-Atom Exchange Effects in bcc3 HeDocument3 pagesTwo-, Three-, and Four-Atom Exchange Effects in bcc3 HezittoxNo ratings yet
- Financial management project report for cement factoryDocument106 pagesFinancial management project report for cement factoryAksh KhandelwalNo ratings yet
- Understand The Standardization Protocol For Iot Understand The Concepts of Web of Things. Understand The Concepts of Cloud of Things With Understand The Basic Concepts of Aspect OrientedDocument2 pagesUnderstand The Standardization Protocol For Iot Understand The Concepts of Web of Things. Understand The Concepts of Cloud of Things With Understand The Basic Concepts of Aspect OrientedShanthi GanesanNo ratings yet
- Product Manual For Resilient Seated Cast Iron Air Relief Valves For Water Works Purposes ACCORDING TO IS 14845: 2000Document5 pagesProduct Manual For Resilient Seated Cast Iron Air Relief Valves For Water Works Purposes ACCORDING TO IS 14845: 2000Tanmoy DuttaNo ratings yet
- Modern Scoring BRASS Manual1Document25 pagesModern Scoring BRASS Manual1Pepe ChorrasNo ratings yet
- Discussion QuestionsDocument45 pagesDiscussion QuestionsSriRahayuNo ratings yet
- Rex - O. Ed. Wagner - W-Waves - BiocommDocument13 pagesRex - O. Ed. Wagner - W-Waves - BiocommLeon BlažinovićNo ratings yet
- Info Iec62271-1Document28 pagesInfo Iec62271-1RichardLemusNo ratings yet
- Projectile LoomDocument23 pagesProjectile Loommehedi111560% (5)
- POster EGU PDFDocument1 pagePOster EGU PDFAsaf Aguilar LemaNo ratings yet
- Measuring Propeller Drop With The Help of Poker GaugeDocument2 pagesMeasuring Propeller Drop With The Help of Poker Gaugeas100% (1)
- Chapter 5 Lennard Jones PotentialDocument6 pagesChapter 5 Lennard Jones PotentialMuhamad RayhanNo ratings yet
- Unit Vi: Classification and PredictionDocument29 pagesUnit Vi: Classification and PredictionpalaniappanNo ratings yet
- IT407 Knowledge EngineeringDocument2 pagesIT407 Knowledge EngineeringVidya ANo ratings yet
- A2 Biopharm MetalDocument28 pagesA2 Biopharm MetalThanh Nghị BùiNo ratings yet
- Tomescu PDFDocument353 pagesTomescu PDFLuís Farias100% (3)