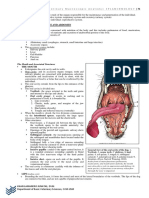
Professional Documents
Culture Documents
(Dapus 21) v086p0F190
Uploaded by
Rohmantuah_Tra_1826Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(Dapus 21) v086p0F190
Uploaded by
Rohmantuah_Tra_1826Copyright:
Available Formats
ORIGINAL ARTICLE
Can transcutaneous bilirubinometry reduce the need for
blood tests in jaundiced full term babies?
L Briscoe, S Clark, C W Yoxall
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Arch Dis Child Fetal Neonatal Ed 2002;86:F190F192
Background: Previous studies have suggested that transcutaneous bilirubinometry (TcB) may provide
a useful method for screening for significant jaundice, thereby reducing unnecessary blood tests. These
studies have not allowed an estimation of the magnitude of such a benefit.
Objectives: To evaluate the accuracy of TcB as a method of determining the need for serum bilirubin
(SBR) measurements in full term babies and to quantify the magnitude of any benefit.
Subjects: Babies born at more than 34 weeks gestation who had not previously been exposed to photo-
therapy and were requiring blood sampling in the first week of life.
Method: TcB measurements were made at the same time as blood sampling. SBR was measured in all
blood samples. For jaundiced babies, the ability of TcB to detect significant jaundice (SBR > 249
mol/l) was evaluated.
Results: There was a correlation between SBR and TcB measurements (n = 303, r = 0.76,
p < 0.0001), but the 95% prediction interval for SBR from TcB was wide ( 88.3 mol/l). For the 285
jaundiced babies, the area under the receiver operator characteristic curve was 0.89. A TcB value of
18 detected significant jaundice with a sensitivity of 100% and a specificity (95% confidence interval)
of 45% (39% to 51%). If blood samples had only been taken from babies with a TcB value greater than
18, the number of samples taken would have been reduced by 34%.
Conclusions: SBR cannot be measured accurately by TcB. However, TcB measurements can be used
to determine the need for blood sampling in jaundiced babies and will reduce the number of blood
samples taken. Recent improvements in TcB may improve the performance of this method.
T
he decision to measure serum bilirubin (SBR) in a
jaundiced baby is subjective and depends on the junior
doctor or midwife making a clinical assessment of the
colour of the babys skin. Physiological jaundice is of course
common; 65% of babies have SBR concentrations of 85 mol/l
or more, with the peak concentration occurring on day 5 of
life,
1
but only 6% of newborn infants develop hyperbilirubi-
naemia (> 220 mol/l).
2
There is a signicant error in the
clinical assessment of neonatal jaundice, and many of the
measurements made are below the threshold for treatment.
Measuring SBR in babies with a concentration below the
treatment threshold involves unnecessary blood sampling.
The wait for the result may also unnecessarily delay discharge
of both the mother and baby from the hospital. Transcutane-
ous bilirubinometry (TcB), using the Minolta Air Shields
jaundice meter JM-102 (Hill-Rom-Airshields, Ashby de la
Zauch, UK), has been proposed as a method of determining
whether a baby requires SBR to be measured. It is a
non-invasive optical method, which assesses jaundice by
measuring the absorbance of reected light in the blue and
green wavebands to measure the yellowness of the skin.
3
Several publications have described TcB as a useful screen-
ing method for detecting clinically signicant jaundice.
Strange and Cassady
4
reviewed all such studies before 1985
and reported sensitivity values ranging from 83% to 100%,
with specicity values of 3497%. Most of these studies were
awed by the inclusion of multiple TcB measurements from
some infants. In addition, all of these studies were small, with
a range of 8129 total measurements. The total number of
measurements made in babies with clinically signicant
bilirubin concentrations in each study was very small, and this
also reduces the value of each of these studies as an evaluation
of TcB as a screening method. Similar studies have been
reported since 1985
5 6
with similar results and similar
limitations. Nonetheless, one American group has reported a
signicant reduction in SBR measurements made in their
institution since the introduction of TcB measurements.
7
Suckling et al
8
reported the use of the bilirubinometer to
identify babies with hyperbilirubinaemia in a UK population
of full term babies. Unfortunately, in that study, SBR was only
measured in those babies who had a TcB measurement above
the threshold value, and therefore the false negative rate could
not be determined.
To dene the most effective cut off value for a screening test,
it is necessary to construct a receiver operator characteristic
(ROC) curve. Lin et al
9
have done this for TcB in a Chinese
population and reported that the best performance they could
achieve was a sensitivity of 84% with a specicity of 84%.
The primary aim of our study was to develop an ROC curve
for TcB measurement to detect hyperbilirubinaemia in term
babies in the rst week of life, and also to identify the most
informative value of TcB in terms of sensitivity and specicity.
We also wished to estimate the magnitude of the reduction in
SBR measurements in our hospital that we could achieve by
adopting TcB as a screening method in term babies with clini-
cally detectable jaundice.
METHODS
This prospective observational study was carried out at Liver-
pool Womens Hospital, a regional teaching hospital with
about 6000 deliveries per annum.
Babies on the postnatal wards with a gestation of more than
34 weeks who were having blood taken for any reason were
eligible to enter the study unless they had previously received
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Abbreviations: SBR, serum bilirubin; TcB, transcutaneous
bilirubinometry
See end of article for
authors affiliations
. . . . . . . . . . . . . . . . . . . . . . .
Correspondence to:
Dr Yoxall, Liverpool
Womens Hospital, Crown
Street, Liverpool L8 7SS,
UK; Bill.Yoxall@
lwh-tr.nwest.nhs.uk
Accepted
22 November 2001
. . . . . . . . . . . . . . . . . . . . . . .
F190
www.archdischild.com
phototherapy. Most of the babies were having blood taken for
SBR measurement to evaluate clinical jaundice, but some
non-jaundiced babies were also included. Measurements from
non-jaundiced babies were used to investigate the correlation
between the two methods, but not for assessing the effective-
ness of TcB as a screening test. Because skin coloration is
known to affect the TcB measurement, the ethnic origin of
each baby was recorded.
After informed written consent was obtained from the par-
ents, blood was taken for SBR. TcB measurements were made
by the phlebotomist concurrently with the blood test. The
mean of three TcB measurements from the babys forehead
was taken. Each eligible infant had only one paired measure-
ment.
SBR was measured in the laboratory using a standard diazo
method (Cobas Integra 700; Roche Diagnostics, Welwyn Gar-
den City, Herts, UK). This method is known to be prone to
interference by high haemoglobin concentrations, but this
becomes less signicant at higher bilirubin concentrations.
This method is therefore not ideal for measuring SBR, but
these measurements are those used by staff to determine
management of jaundiced babies in everyday clinical practice.
This was a pragmatic study to determine whether TcB meas-
urements would alter clinical practice.
The indication for starting phototherapy in this group of
babies in our hospital is an SBR concentration of > 250 mol/l
on the second day of life, or > 300 mol/l thereafter. For the
purposes of analysis, the denition of hyperbilirubinaemia
was an SBR concentration > 249 mol/l.
Sample size
The number of babies needed to produce an adequate ROC
curve was estimated using the method of Schafer.
10
This was
an estimate because of the paucity of data available to allow
calculation of a true sample size. Assuming a true sensitiv-
ity of 95%, = 0.05, = 0.1, = 1, and a minimum required
sensitivity of 90%, a total of 452 babies was required.
Analysis
The relation between TcB values and all SBR measurements
was investigated using simple linear regression analysis.
A ROC curve was constructed to determine which TcB value
had the greatest overall predictive power in babies in whom
the blood test had been performed to evaluate clinical
jaundice. The lowest TcB value to give 100% sensitivity for
detecting signicant jaundice in these babies was also
determined, and the effect of using this value on the number
of SBR measurements was determined.
RESULTS
Consent was obtained for a total of 469 babies to enter the
study. Paired measurements were available for only 303
babies. In the other 166 babies, paired data were not available
because the decision to measure SBR was cancelled (n = 69),
because an inadequate sample of blood was collected for SBR
measurement (n = 49), or because the bilirubinometer was
not available (n = 48). Although we had not obtained our
intended number of paired measurements, recruitment into
the study was terminated on the basis of an interim analysis
of the results at that point which showed that our aims had
been achieved. In 285 of the 303 babies with paired measure-
ments, SBR was measured to evaluate their jaundice, and, in
the other 18 babies, SBR was measured in blood samples taken
for other purposes.
The median (range) values for birth weight, gestation, and
age were 3267 (18005008) g, 39 (3442) weeks, and 3 (013)
days respectively. Most (287) of the subjects were white. This
is representative of the total population within our hospital,
where 94% of the babies born are white.
The SBR concentrations were normally distributed (g 1).
Most measurements fell between 150 and 300 mol/l.
Eighteen babies had concentrations below 100 mol/l, and 10
had concentrations above 300 mol/l.
There was a signicant correlation between SBR and TcB
measurements (n = 303, r = 0.76, p < 0.0001; g 2). The pre-
diction interval for SBR from TcB measurement was wide,
however. The standard deviation about the regression line was
45.05 mol/l, giving a 95% prediction interval of 88.3 mol/l.
Of the 285 babies for whom SBR was measured to evaluate
clinically apparent jaundice, 53 had signicant jaundice (SBR
> 249 mol/l). The area under the ROC curve was 0.89 (g 3).
Analysis of the ROC curve showed that the TcB value that
detected signicant jaundice (SBR > 249 mol/l) with the
greatest predictive value was 19.9. This gave a sensitivity (95%
condence interval (CI)) of 86%(81%to 89%) and a specicity
(95% CI) of 78% (73% to 83%). However, for clinical purposes,
this is unsatisfactory as a sensitivity of 100% is needed. The
TcB value that gave 100% sensitivity was 18, which gave a spe-
cicity (95% CI) of 45% (39% to 51%).
Figure 1 Distribution of serum bilirubin measurements.
120
100
80
60
40
20
0
Serum bilirubin concentration (mol/l)
N
u
m
b
e
r
o
f
i
n
f
a
n
t
s
<50
3
50
100
15
101
150
70
151
200
102
201
250
63
251
300
40
301
351
8
351
409
2
Figure 2 Correlation between transcutaneous bilirubinometry (TcB)
and serum bilirubin (SBR) measurements.
500
400
300
200
100
0
30
TcB
n = 303, r = 0.76, p < 0.0001
SBR = 0.039(TcB) 11.3
SD = 45.05
S
B
R
(
m
o
l
/
l
)
5 25 20 15 10
Figure 3 Receiver operator characteristics curve of transcutaneous
bilirubin to detect serum bilirubin > 249 mol/l.
1.00
0.75
0.50
0.25
0.00
1.00
1 Specificity
S
e
n
s
i
t
i
v
i
t
y
0.00 0.75 0.50 0.25
Transcutaneous bilirubinometry for screening for jaundice F191
www.archdischild.com
In our cohort of 285 babies having blood tests to evaluate
their jaundice, if we had taken blood from only babies with a
meter reading of 18 or more, we would have prevented 104
blood tests, a reduction of 34%.
DISCUSSION
Although there is a close correlation between TcB and SBR
measurements, the prediction interval for SBR from TcB is
wide, and TcB cannot be used to measure SBR. However, the
relation between TcB values and SBR allows TcB to be used as
a screening tool for babies requiring SBR measurement.
In our unit, if TcB measurements were used to determine
the need for SBR measurements in babies in whom there is
clinical concern about signicant jaundice, then the number
of blood tests would be reduced by 34%. It should be remem-
bered that the predictive ability of any test is reduced when it
is applied to a population with a lower prevalence of the con-
dition being tested for. The use of TcB as a screening test in
babies in whom there is no clinical concern about jaundice
cannot therefore be justied on the basis of this study.
Furthermore, because of differences in population character-
istics and laboratory methods of assaying bilirubin, the
manufacturers of the transcutaneous bilirubinometer recom-
mend that each unit should develop a cut off point for use
with this instrument that is appropriate for its population. The
results of our study are therefore not directly transferable to
other units. The babies in this study were mostly white. In a
population with a higher proportion of non-white babies, the
performance of TcB measurements is unlikely to give the same
performance, as skin pigmentation is known to affect the TcB
reading.
6 11
Recent developments in the eld of TcB include the
development of newer bilirubinometers using more sophisti-
cated optical technology, which may allow measurement of
bilirubin rather than simply measuring skin yellowness.
12 13
These may eventually prove to be more accurate methods for
non-invasive screening for jaundice, but further published
data are awaited.
It could be argued that TcB is a better method of measuring
jaundice than SBR as it measures tissue bilirubin deposition
rather than concentration in the blood. Unfortunately, there
are no epidemiological data to support the use of TcB rather
than SBR measurements. The relation between bilirubin con-
centration in the skin and in the central nervous system is not
known, so the value of TcB in assessing tissue bilirubin
concentration on the other side of the blood-brain barrier also
remains unknown. In reality, most paediatricians are likely to
adopt a pragmatic approach and rely on SBR measurements,
with which there has been extensive experience developed
over many years.
There is no consensus about when to evaluate and treat
neonatal jaundice. Most of the scientic data relating to safe
levels of hyperbilirubinaemia have been obtained from
uncontrolled observations of babies with haemolytic disease
of the newborn. However, these observations were largely
made over 30 years ago when many of the babies had associ-
ated prematurity and asphyxia and were often exposed to the
antibiotic streptomycin. All of these factors are known to
potentiate bilirubin toxicity, and the safe level of bilirubin
for haemolytic disease in the modern era is not clearly known.
There is also a paucity of scientic evidence as to the safe level
of bilirubin in healthy term babies without haemolysis.
Despite this, most neonatal authorities believe that there is
still a risk to term babies with non-haemolytic jaundice and,
although there is no clear consensus on a denition, all would
recommend treatment of hyperbilirubinaemia.
Treatment of jaundice will remain a common intervention
in neonatal medicine, and this will be based on SBR measure-
ments. Clinical assessment of jaundice is inaccurate and
results in a signicant number of blood tests in otherwise well
babies. The number of these blood tests could be reduced, with
benet to the babies and potential cost savings, by using TcB
as a screening tool
. . . . . . . . . . . . . . . . . . . . .
Authors affiliations
L Briscoe, S Clark, C W Yoxall, Liverpool Womens Hospital, Crown
Street, Liverpool L8 7SS, UK
REFERENCES
1 Maisels MJ. Neonatal jaundice. In: Sinclair JC, Bracken MB, eds.
Effective care of the newborn. Oxford: Oxford University Press,
1993:507562.
2 Dai J, Parry DM, Krahn J. Transcutaneous bilirubinometry: its role in the
assessment of neonatal jaundice. Clin Biochem 1997;30:19.
3 Yamamuchi Y, Yamanishi A, Yamanouchi I. Transcutaneous monitoring
of bilirubin Minolta jaundice meter. In: Brans YW, Hay WW, eds.
Physiological monitoring and instrument diagnosis in perinatal and
neonatal medicine. Cambridge: Cambridge University Press, 1995.
4 Strange M, Cassady G. Neonatal transcutaneous bilirubinometry. Clin
Perinatol 1985;12:5162.
5 Schumacher RE, Thornberry JM, Gutcher GR. Transcutaneous
bilirubinometry: a comparison of old and new methods. Pediatrics
1985;76:1014.
6 Linder N, Regev A, Gazit G, et al. Noninvasive determination of
neonatal hyperbilirubinaemia: standardisation for variation in skin color.
Am J Perinatol 1994;3:2235.
7 Maisels MJ, Kring E. Transcutaneous bilirubinometry decreases the need
for serum bilirubin measurements and saves money. Pediatrics
1997;99:599601.
8 Suckling RJ, Laing IA, Kirk JM. Transcutaneous bilirubinometry as a
screening tool for neonatal jaundice. Scot Med J 1995;40:1415.
9 Lin YJ, Ju SN, Lin CH. The clinical application of transcutaneous
bilirubinometry in full term Chinese infants. Acta Paediatr Sin
1993;34:6976.
10 Schafer, H. Constructing cut-off point for a quantitative diagnostic test.
Stat med 1989;8:138191.
11 Goldman SL, Penalver A, Penaranda R. Jaundice meter: evaluation of
new guidelines. J Pediatr 1982;101:2536.
12 Tayabe R, Gribetz D, Gribetz I, et al. Noninvasive estimation of serum
bilirubin. Pediatrics 1988;102:E28.
13 Bhutani VK, Gourley GR, Adler SA, et al. Noninvasive measurement of
total serum bilirubin in a multiracial predischarge newborn population to
assess the risk of severe hyperbilirubinaemia. Pediatrics 2000;106:e17.
F192 Briscoe, Clark, Yoxall
www.archdischild.com
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Volpe's Understanding EvolutionDocument303 pagesVolpe's Understanding EvolutionAnonymous kg7YBMFH100% (1)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Mob Ot 31753002739420Document276 pagesMob Ot 31753002739420fabriziozaraNo ratings yet
- Eysenck Personality TheoryDocument11 pagesEysenck Personality TheoryKarthika RsNo ratings yet
- QA Lesson Plan L3 First Aid at Work RQF - Including Catastrophic BleedingDocument41 pagesQA Lesson Plan L3 First Aid at Work RQF - Including Catastrophic BleedingPhysical Education Scotland88% (8)
- (Brawer) Prostate Specific AntigenDocument342 pages(Brawer) Prostate Specific Antigenseeranga_raghuNo ratings yet
- Flavone Synthesis ThesisDocument300 pagesFlavone Synthesis ThesisJamal Rafique100% (1)
- The Smoking Scare De-Bunked William T WithbyDocument61 pagesThe Smoking Scare De-Bunked William T WithbyPietje PukNo ratings yet
- Daftar PustakaDocument1 pageDaftar PustakaRohmantuah_Tra_1826No ratings yet
- Daftar PustakaDocument4 pagesDaftar PustakaRohmantuah_Tra_1826No ratings yet
- Bartholin's Abscess Caused by Hypermucoviscous Klebsiella PneumoniaeDocument3 pagesBartholin's Abscess Caused by Hypermucoviscous Klebsiella PneumoniaeRohmantuah_Tra_1826No ratings yet
- Indinavir-Induced Nephrolithiasis Three and One-Half Years After Cessation of Indinavir TherapyDocument3 pagesIndinavir-Induced Nephrolithiasis Three and One-Half Years After Cessation of Indinavir TherapyRohmantuah_Tra_1826No ratings yet
- Identity Development Throughout The Lifetime - An Examination of EDocument12 pagesIdentity Development Throughout The Lifetime - An Examination of ERohmantuah_Tra_1826No ratings yet
- Treatment of Iatrogenic Cushing's Syndrome: Questions of Glucocorticoid WithdrawalDocument10 pagesTreatment of Iatrogenic Cushing's Syndrome: Questions of Glucocorticoid WithdrawalRohmantuah_Tra_1826No ratings yet
- Immunology of PreeclampsiaDocument13 pagesImmunology of PreeclampsiaRohmantuah_Tra_1826No ratings yet
- Management of Neonatal Jaundice - Depkes MalaysiaDocument5 pagesManagement of Neonatal Jaundice - Depkes MalaysiaRohmantuah_Tra_1826No ratings yet
- NIH Public Access: Author ManuscriptDocument13 pagesNIH Public Access: Author ManuscriptRohmantuah_Tra_1826No ratings yet
- PROM Dilemmas: Choosing A Strategy, Knowing When To Call It QuitsDocument8 pagesPROM Dilemmas: Choosing A Strategy, Knowing When To Call It QuitsRohmantuah_Tra_1826No ratings yet
- Module 5 Anatomy I Splanchnology 1Document17 pagesModule 5 Anatomy I Splanchnology 1neannaNo ratings yet
- Intraspecific CompetitionDocument2 pagesIntraspecific CompetitionZainab MahmudNo ratings yet
- Acs Graphene Oxide DatasheetDocument7 pagesAcs Graphene Oxide Datasheet9semNo ratings yet
- Bio 2Document9 pagesBio 2triwicak15No ratings yet
- 12 Chem CH 10 MCQSDocument12 pages12 Chem CH 10 MCQSSaran.kNo ratings yet
- 1,4 ButanediolDocument6 pages1,4 ButanediolVictor VikeneNo ratings yet
- Reflection On Living Soil - Manaloto - II-24Document1 pageReflection On Living Soil - Manaloto - II-24Ren Manaloto0% (1)
- Een House Monitoring and Spray Water SystemDocument2 pagesEen House Monitoring and Spray Water SystemDinesh KumarNo ratings yet
- Biodiversity: Mr. Gabino E. Santos, MS.CDocument62 pagesBiodiversity: Mr. Gabino E. Santos, MS.CJan MichaelNo ratings yet
- Progress Test 2Document5 pagesProgress Test 2Ngát Phạm HồngNo ratings yet
- Villacís2019 Article EfficacyOfPulsed-xenonUltravioDocument6 pagesVillacís2019 Article EfficacyOfPulsed-xenonUltravioByron JimenezNo ratings yet
- Craetagusarticle PDFDocument7 pagesCraetagusarticle PDFKatty AcostaNo ratings yet
- Impact of Postharvest Salicylic Acid and Jasmonic Acid Treatments On Quality of "Crimson Seedless" Grapes During Cold Storage and Shelf LifeDocument8 pagesImpact of Postharvest Salicylic Acid and Jasmonic Acid Treatments On Quality of "Crimson Seedless" Grapes During Cold Storage and Shelf LifeFadhilah SurotoNo ratings yet
- Encyclopedia ES HealthDocument9 pagesEncyclopedia ES HealthGantsooj BNo ratings yet
- Assessment Diagnosis Outcome Intervention Rationale EvaluationDocument7 pagesAssessment Diagnosis Outcome Intervention Rationale EvaluationArmie Joy Embat CariazoNo ratings yet
- Heritability of Angular Leaf Spot Resistance in Populations of Common Bean Developed Using g5686, Mexico 54, Amendoim and Bat 332, As Donor ParentsDocument5 pagesHeritability of Angular Leaf Spot Resistance in Populations of Common Bean Developed Using g5686, Mexico 54, Amendoim and Bat 332, As Donor ParentsijsidonlineinfoNo ratings yet
- Enteropathogenic Escherichia Coli RESISTEN ANTIBIOTIK: Infektifitas Fage Litik Dari Limbah Cair Rumah Tangga TerhadapDocument61 pagesEnteropathogenic Escherichia Coli RESISTEN ANTIBIOTIK: Infektifitas Fage Litik Dari Limbah Cair Rumah Tangga TerhadapAisyah AminiNo ratings yet
- Ielts Reading Practice Test 65 With Answers PDFDocument14 pagesIelts Reading Practice Test 65 With Answers PDFSri KarthickNo ratings yet
- Eggs Fase 1Document3 pagesEggs Fase 1Gustav MolMedNo ratings yet
- Clinical EMG ALSDocument7 pagesClinical EMG ALSAbul Shamsud DoulahNo ratings yet
- Ethylhexylglycerin Safety ToxicologyDocument17 pagesEthylhexylglycerin Safety Toxicologybfh83730No ratings yet
- Genul StaphylococcusDocument28 pagesGenul StaphylococcusAna Maria RusuNo ratings yet
- Water in The Human BodyDocument8 pagesWater in The Human BodyPaula AbadNo ratings yet