Professional Documents
Culture Documents
Cre-IRES-GFP Retrovirus
Uploaded by
AlleleBiotechOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cre-IRES-GFP Retrovirus
Uploaded by
AlleleBiotechCopyright:
Available Formats
Expression Ready
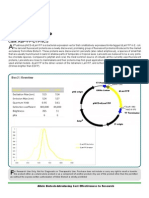
Cre-IRES-GFP Retrovirus
T he bacterial phage P1 Cre/lox
recombination system mediates
DNA excision, integration, inversion
Allele Biotech provides custom
retrovirus or lentivirus packaging
services using proprietary
AmpR 5' LTR
and translocation in a sequence technologies that are unique and
specific manner involving only two highly efficient, yielding 108 to 109
genetic components: the 38-kDa TU/ml, without any concentrating
Cre (cyclization recombination) steps (US patents 6,207,455 and
Cre
recombinase and two 34 bp loxP 6,531,123). These technologies and
(locus of X-over of P1) sites. The optimal operation procedures enable
most efficient Cre/lox reaction is DNA Allele Biotech to package viruses at EBNA pCHAC
pBMN-Cre-IRES-GFP IRES
excision from a “floxed” locus (flanked < 1% of the market price in certain
by loxP) and this reaction has been categories on per million particle GFP
frequently applied for mouse genome basis. Right now, based on this strong
modification. platform, Allele Biotech offers pre-
3' LTR
Viral vectors are widely used packaged retroviral particles ready to
vehicles to bring genetic material infect target cells for Cre/loxP system. pUC
PGK
inside eukaryotic cells. Compared PuroR polyA
to transfection methods such as The Expression Ready Cre-IRES-
cationic lipids, electroporation, or GFP retroviral particles encode Cre
microinjection, induction with viral for Cre/LoxP expirments. Retrovirus
particles is generally more efficient also expresses GFP as a marker. Viral
if an appropriate viral system with Vector map shown below:
correct tropism is selected.
Protocols
Storage: -80°C. Avoid repeated freeze thaw cycles
Related Products
Steps:
1. Plate target cells to 40-60% confluence in a 24-well plate before ♦ pCHAC Retroviral Vectors
infection. - mWasabiGFP Control
2. Place virus stock (virus titer: 1x108 IU/ml) in a 37°C water bath and - LacZ Control
before totally thaw transfer onto ice immediately.
3. Make a serial dilution of 3-4 different multiplicity of infection ♦Gryphon Retroviral Packaging
(MOI = infectious units/ cell number) to test the target cells. Example 1x104 Cell Lines
TE671 cells in each well of a 24-well plate: - Ampho Cell
10MOI 20MOI 30MOI 60MOI - Eco Cell
Culture Medium (ul): 198 197 195 189 ♦Gryphon Packaging Cell
Polybrene (2mg/ml): 1 1 1 1 Selection Medium
Gryphon Hexamethrine Bromide for
Virus (ul): 1 2 4 10 viral infection enhancement
Total volume (ul): 200 200 200 200
4. Tilt the plate gently every 30 min. After 3hrs, add 1 ml fresh medium.
5. After 48-72 hrs, evaluate GFP expression by using fluorescence
microscope or flow cytometry.
S afety Issues: Retroviral vectors should be handled using
NIH BSL-2 safety guidelines. For more information, please
F or Research Use Only. Not for Diagnostic or
Therapeutic Use.
Purchase does not include or carry any right to resell or
see Biosafety in Microbiological and Biomedical Laboratories
transfer this product either as a stand-alone product or as
(4th edition) which is available on the Web sites of the National
a component of another product. Any use of this product
Institutes of Health at http://bmbl.od.nih.gov.
other than the permitted use without the express written
authorization of Allele Biotech is strictly prohibited
Allele Biotech-Introducing Cost Effectiveness to Research
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Lesson Plan For Demo TeachingDocument6 pagesLesson Plan For Demo Teachingjanice alquizar100% (6)
- Practical BotanyDocument123 pagesPractical BotanyKasraSr100% (12)
- Peperiksaan Pertengahan Tahun Science DLP 2017Document6 pagesPeperiksaan Pertengahan Tahun Science DLP 2017Laila Maisarah71% (7)
- M13KO7 Helper PhageDocument1 pageM13KO7 Helper PhageAlleleBiotechNo ratings yet
- Anti-RFP (5F8) Monoclonal AntibodyDocument1 pageAnti-RFP (5F8) Monoclonal AntibodyAlleleBiotechNo ratings yet
- Modified UTPDocument1 pageModified UTPAlleleBiotechNo ratings yet
- High Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionDocument1 pageHigh Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionAlleleBiotechNo ratings yet
- Modified CTPDocument1 pageModified CTPAlleleBiotechNo ratings yet
- Custom Sub Cloning ServiceDocument1 pageCustom Sub Cloning ServiceAlleleBiotechNo ratings yet
- Anti-RFP (3F5) Monoclonal AntibodyDocument1 pageAnti-RFP (3F5) Monoclonal AntibodyAlleleBiotechNo ratings yet
- High Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionDocument1 pageHigh Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionAlleleBiotechNo ratings yet
- PNCS dLanYFPDocument1 pagePNCS dLanYFPAlleleBiotechNo ratings yet
- Mwasabi PCR Template For IVTDocument1 pageMwasabi PCR Template For IVTAlleleBiotechNo ratings yet
- 3' DG-CPGDocument1 page3' DG-CPGAlleleBiotechNo ratings yet
- ChromoTek RFP BoosterDocument1 pageChromoTek RFP BoosterAlleleBiotechNo ratings yet
- Luciferase PCR Template For IVTDocument1 pageLuciferase PCR Template For IVTAlleleBiotechNo ratings yet
- Allele AgaroseDocument1 pageAllele AgaroseAlleleBiotechNo ratings yet
- mClavGR2 Fusion VectorsDocument3 pagesmClavGR2 Fusion VectorsAlleleBiotechNo ratings yet
- pmTFP1 ClathrinDocument4 pagespmTFP1 ClathrinAlleleBiotechNo ratings yet
- 3' DC-CPGDocument1 page3' DC-CPGAlleleBiotechNo ratings yet
- 3' DT-CPGDocument1 page3' DT-CPGAlleleBiotechNo ratings yet
- 3'-Thiol-Modifier C6 S-S CPGDocument1 page3'-Thiol-Modifier C6 S-S CPGAlleleBiotechNo ratings yet
- Gryphon Selection MediumDocument1 pageGryphon Selection MediumAlleleBiotechNo ratings yet
- 3'-Amino-Modifier C7 CPGDocument1 page3'-Amino-Modifier C7 CPGAlleleBiotechNo ratings yet
- Anatomy AND Physiology: Anatomical PositionDocument9 pagesAnatomy AND Physiology: Anatomical PositionRiyalynkate DellomesNo ratings yet
- Bianda Axanditya 22010110130181 Bab2ktiDocument10 pagesBianda Axanditya 22010110130181 Bab2ktimeiutaNo ratings yet
- CEPHalometryDocument107 pagesCEPHalometrydisha 146jandialNo ratings yet
- Lecture On Science and Technology and Society Notes General Paper 2019Document8 pagesLecture On Science and Technology and Society Notes General Paper 2019DLNo ratings yet
- CPB 30103 Biochemical Engineering UniKL MICET Experiment 4: Determination of Bacterial Loads Viable Cell Counts Full Lab ReportDocument12 pagesCPB 30103 Biochemical Engineering UniKL MICET Experiment 4: Determination of Bacterial Loads Viable Cell Counts Full Lab ReportSiti Hajar Mohamed100% (2)
- Solid Lipid Nanoparticles (SLN)Document33 pagesSolid Lipid Nanoparticles (SLN)reyviNo ratings yet
- Extended BodyDocument5 pagesExtended BodyHyphae ProjectNo ratings yet
- The Learners Demonstrate Understandin G Of... The Learners Should Be Able To..Document3 pagesThe Learners Demonstrate Understandin G Of... The Learners Should Be Able To..Sab Ibarreta100% (2)
- List of Subjects CodeDocument29 pagesList of Subjects CodeSnehajit TaleNo ratings yet
- Behold The Mighty DinosaurDocument65 pagesBehold The Mighty DinosaurLuis Fernandez100% (3)
- Progress in PD-1 - PD-L1 Pathway Inhibitors - From Biomacromolecules To Small MoleculesDocument30 pagesProgress in PD-1 - PD-L1 Pathway Inhibitors - From Biomacromolecules To Small MoleculesMario Andres JuradoNo ratings yet
- Crone Dahl 2012 NRNDocument16 pagesCrone Dahl 2012 NRNFernandaNo ratings yet
- Hematology and Coagulation Controls: Your Quality Goals RealizedDocument15 pagesHematology and Coagulation Controls: Your Quality Goals RealizedBenn BasilNo ratings yet
- Erotic MassageDocument2 pagesErotic MassageKarugu MartinNo ratings yet
- Seminar 23 Feb 2013 Grand Zuri - ANTI AGING - Dr. Dr. Amarullah H. Siregar, PHDDocument49 pagesSeminar 23 Feb 2013 Grand Zuri - ANTI AGING - Dr. Dr. Amarullah H. Siregar, PHDUmmuNo ratings yet
- Chemistry of Amino AcidDocument15 pagesChemistry of Amino AcidWay FarerNo ratings yet
- Dating of FossilsDocument23 pagesDating of FossilsshieldNo ratings yet
- Diagnostic Test Science 10Document8 pagesDiagnostic Test Science 10Pilar Angelie Palmares VillarinNo ratings yet
- Inter 1 Biology Success SeriesDocument8 pagesInter 1 Biology Success Seriesashfaq4985No ratings yet
- Botany (Week 1-5) Merged PDFDocument287 pagesBotany (Week 1-5) Merged PDFLisa MuthiniNo ratings yet
- Medical Biotechnology: A Resource Guide For Biotechnology Club SponsorsDocument39 pagesMedical Biotechnology: A Resource Guide For Biotechnology Club Sponsorsim_mogerzNo ratings yet
- Congenital Abnormalities IDMDocument9 pagesCongenital Abnormalities IDMkatskoufNo ratings yet
- Dr. Diah Rumekti hadiatiAPCGS JogjaDocument25 pagesDr. Diah Rumekti hadiatiAPCGS JogjacirererereNo ratings yet
- Inhibitory Control Over Action and MemoryDocument11 pagesInhibitory Control Over Action and MemoryMaryam TaqaviNo ratings yet
- SOL BIO Anatomical Evidence of Evolution Henry PriceDocument7 pagesSOL BIO Anatomical Evidence of Evolution Henry PriceHenry PriceNo ratings yet
- Guía Examen Inglés 3Document9 pagesGuía Examen Inglés 3LUIS JOAQUIN AYALA GALLARDONo ratings yet
- MT DNA AND SEQUENCINGDocument2 pagesMT DNA AND SEQUENCINGNived K KNo ratings yet