0% found this document useful (0 votes)
28 views5 pagesMineral Composition and Percentages
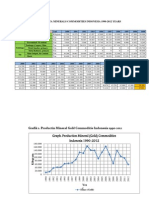
The document discusses the percentages of elements in various minerals including native elements, sulfides, oxides and hydroxides, carbonates, sulphates, and silicates. It provides the chemical formula, atomic mass, and percentage composition of elements in minerals such as gold, sulfur, bismuth, chalcopyrite, pyrite, geothite, spinel, corundum, dolomite, calcite, barite, gypsum, orthoclase, serpentine, and tourmaline. Percentage compositions are calculated using atomic masses and weights.
Uploaded by
Evan Sutikno JuntakCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
28 views5 pagesMineral Composition and Percentages
The document discusses the percentages of elements in various minerals including native elements, sulfides, oxides and hydroxides, carbonates, sulphates, and silicates. It provides the chemical formula, atomic mass, and percentage composition of elements in minerals such as gold, sulfur, bismuth, chalcopyrite, pyrite, geothite, spinel, corundum, dolomite, calcite, barite, gypsum, orthoclase, serpentine, and tourmaline. Percentage compositions are calculated using atomic masses and weights.
Uploaded by
Evan Sutikno JuntakCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd