0% found this document useful (0 votes)
68 views2 pagesData Processing and Analysis Guide
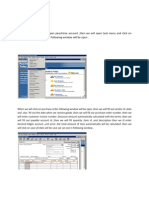
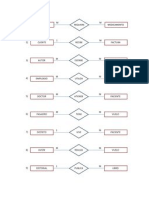
This document contains 3 tables showing observations from an experiment using a continuous stirred tank reactor. Table 4.1 and 4.2 show the standardization of NaOH and HCl solutions. Table 4.3 shows the observation table with the volume of HCl added, flow rate, volume of NaOH used for titration and initial and final burette readings at different flow rates of HCl. The reactions involved are the neutralization of oxalic acid by NaOH, HCl by Na2CO3, and the production of phenolphthalein from a reaction using HCl and Na2CO3 with methyl orange and phenolphthalein as indicators.
Uploaded by
ThornCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
68 views2 pagesData Processing and Analysis Guide
This document contains 3 tables showing observations from an experiment using a continuous stirred tank reactor. Table 4.1 and 4.2 show the standardization of NaOH and HCl solutions. Table 4.3 shows the observation table with the volume of HCl added, flow rate, volume of NaOH used for titration and initial and final burette readings at different flow rates of HCl. The reactions involved are the neutralization of oxalic acid by NaOH, HCl by Na2CO3, and the production of phenolphthalein from a reaction using HCl and Na2CO3 with methyl orange and phenolphthalein as indicators.
Uploaded by
ThornCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd