Debye-Scherrer experiment of diffraction
1). The purpose of the work The work studies the determination of wavelength of the electrons, checking the de Broglie equation, determine the constant of the graphite network. 2).Theory of the work In 1924 Louis de Broglie suggested that beyond the specific properties of small parts, they may have and undulating nature and assumed that the wavelength of a free particle is given by the relation:
(1)
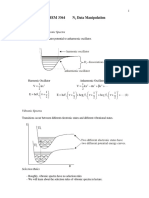
,where: is the wavelength associated to the particle, h is the Plancks constant and p is the particles impulse In the present experiment is proved the undulating nature of the electrons by an electron diffraction experience on a polycrystalline graphite network, experiment known as the DebyeScherrer diffraction. A monochromatic electron beam emitted from a cathode tube is focused by an electromagnetic lens system and fall on a sheet of polycrystalline graphite. The atoms of the graphite are arranged in a crystalline network that acts as a diffraction network for electron, on a fluorescent screen appearing the diffraction figure as two concentric rings (Fig. 1) corresponding to the two network constants and (Fig. 3). The diameter of the concentric rings changes depending on the wavelength of the electrons and thus, depending on the acceleration voltage, as the considerations below. Fig. 1 Schematic representation of diffraction rings. The two rings
with diameters
and
corresponding to the network constants
and
(cf. Fig. 3)
The energy of an electron accelerated in the field of potential energy U is:
(2)
,where: U is the acceleration voltage, e is electric charge of the electron, p is the electrons impulse. Replacing the impulse p from the equation (2):
(3)
expression which prove that the associated wavelength of the electron is determined by the acceleration voltage U. If on a set of crystalline planes falls a monochromatic beam of X-rays or electrons single-charged allegedly having wave nature, each element of the crystal plane acts as a scattering centre generating an elementary spherical wave reflected, the superposition of these
Page 1
�elementary spherical waves generating a reflected wave front. Applying the laws of reflection, the wavelength of reflected wave is the same with the incident wave and the angle of reflection equals the angle of incidence. From the superposition of the reflected waves on successive planes are obtained maxima of interference (constructive interference) if the difference in path: (Fig. 2) is an integer multiple of wavelengths:
(4)
,where d is the distance between two succesive planes, 2 is the angle between the incident and reflected beam ( the angle measured from the plane). The (4) expression is known as the Braggs relation.
Fig.2. Schematic representation of the diffraction Condition of Bragg
Fig.3 The constants of graphite network
In the experiment of this work it is used a polycrystalline material, which is composed of a very large number of crystals (crystallites) arranged irregularly in space. There will always be a few crystals whose orientation satisfies Bragg condition for one wavelength and direction of the incident beam given. The total of reflections produced from these crystallites are in a cone whose axis is given by the direction of the incident beam, such that on the screen located perpendicular to the axis there will appear concentric rings. Important crystal planes for the diffraction figure of this experiment are as Fig. 3, those for which the networks constants are:
Fig.4.Schematic representation of the angle of diffraction . L=13.5 cm (distance between the sheet of graphite and the screen), D is the diameter of the diffractions ring observed on the screen. From the Fig.4 we obtain the relation:
(5)
,where: D is the rings diameter, and L is the distance between the sheet of graphite and screen For small angles :
Page 2
�(6)
Replacing (6) in (4) we obtain, for the first order of diffraction, n=1, the expression for the wavelength associated to the electrons:
(7)
Taking into consideration the expression (3) for the electrons wavelength we obtain for the rings diameter of diffraction the expression:
(8)
where
(9)
is the slope of the line 3).The experimental set-up
The device is composed of:
, slope which depends of the networks constant d. 4. Red connection cable 25 cm 5. Red connection cable 50 cm 6. Red connection cable 100 cm 7. Blue connection cable 100 cm 8. Black connection cable 100 cm
1. Electron diffraction tube 2. High voltage source: 10kV 3. Vernier 4).Data processing The experimental results are written in the table: U(kV)
a.Determine the wavelength of the electrons. From the measured values of the diffraction rings and the values of the constants network , assumed to be known, using the equation (7) we can obtain the experimental wavelength of the electrons. The experimental measurments for corresponding to different voltages are written in the following tables: U(kV) (obtained from equation (7))
2.2 U(kV)
Page 3
�(obtained from equation (7))
b.Check de Broglie relation. The de Broglie relation (1) is checked using the equation (3), where:
The results obtained for the wavelength corresponding to the different voltages applied are written in the table: U(kV)
We observe that the experimental results of the wavelength of the electrons obtained from the diffraction figure and the ones theoretical are in good correspondence. c.Determination of the networks constant of the graphite According to the equation (8), the diameter of the rings of diffraction D depends of the voltage U:
the slope of the line being determined by the value of the networks constant d according to the equation (9). Experimentally, the slopes are determined from the graphic representation of the measured diamteres as function of . Obtaining these slopes from the above graphics, the networks constants are obtained from the equation (9):
Page 4