80% found this document useful (5 votes)
959 views2 pagesCONSORT Checklist
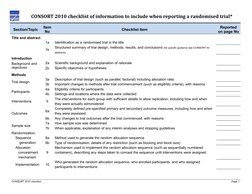
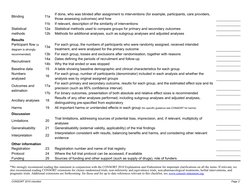
This document is a checklist of information to include when reporting randomized trials based on the CONSORT 2010 guidelines. It includes 25 items across several sections: title and abstract (2 items), introduction (2 items), methods (13 items including trial design, participants, interventions, outcomes), results (7 items including participant flow, recruitment, numbers analyzed, outcomes), and other information (1 item). The checklist aims to improve transparency in trial reporting.
Uploaded by
ziana alawiyahCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
80% found this document useful (5 votes)
959 views2 pagesCONSORT Checklist
This document is a checklist of information to include when reporting randomized trials based on the CONSORT 2010 guidelines. It includes 25 items across several sections: title and abstract (2 items), introduction (2 items), methods (13 items including trial design, participants, interventions, outcomes), results (7 items including participant flow, recruitment, numbers analyzed, outcomes), and other information (1 item). The checklist aims to improve transparency in trial reporting.
Uploaded by
ziana alawiyahCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
- Introduction
- Title and Abstract
- Methods
- Results
- Other Information