100% found this document useful (1 vote)
5K views2 pagesMolecular Geometry and Lewis Structures
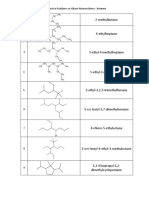
Molecular geometry describes the spatial arrangement of atoms in a molecule. It is determined by the number of electron regions around the central atom. Some key points:
- The number of electron regions, bonding regions, and nonbonding regions determine the molecular geometry.
- Common molecular geometries include linear, bent, trigonal planar, tetrahedral, trigonal pyramidal, and octahedral.
- Lewis structures are a way to represent molecules using dots to represent electrons and lines to represent chemical bonds.
- Steps to draw Lewis structures include counting valence electrons, placing atoms, adding electron pairs, drawing bonds, and assigning formal charges.
Uploaded by
rsleoCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
5K views2 pagesMolecular Geometry and Lewis Structures
Molecular geometry describes the spatial arrangement of atoms in a molecule. It is determined by the number of electron regions around the central atom. Some key points:
- The number of electron regions, bonding regions, and nonbonding regions determine the molecular geometry.
- Common molecular geometries include linear, bent, trigonal planar, tetrahedral, trigonal pyramidal, and octahedral.
- Lewis structures are a way to represent molecules using dots to represent electrons and lines to represent chemical bonds.
- Steps to draw Lewis structures include counting valence electrons, placing atoms, adding electron pairs, drawing bonds, and assigning formal charges.
Uploaded by
rsleoCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd