0% found this document useful (1 vote)
1K views3 pagesAtomic Structure Worksheet
This document contains an atomic structure worksheet that asks students to:
1) Label and draw diagrams of subatomic particles and their locations in an atom.
2) Describe how to determine the number of each subatomic particle in an atom.
3) Fill out a chart with the number of protons, neutrons, and atomic mass of various elements.
4) Draw Bohr models of different atoms and label subatomic particles.
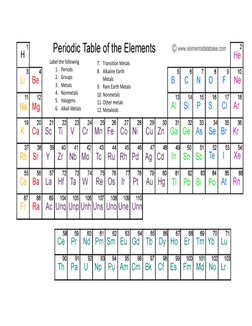
5) Label different groups on the periodic table.
Uploaded by
Rob GamaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (1 vote)
1K views3 pagesAtomic Structure Worksheet
This document contains an atomic structure worksheet that asks students to:
1) Label and draw diagrams of subatomic particles and their locations in an atom.
2) Describe how to determine the number of each subatomic particle in an atom.
3) Fill out a chart with the number of protons, neutrons, and atomic mass of various elements.
4) Draw Bohr models of different atoms and label subatomic particles.
5) Label different groups on the periodic table.
Uploaded by
Rob GamaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Atomic Structure Worksheet
- Atomic Models
- Periodic Table of the Elements