0% found this document useful (0 votes)
1K views5 pagesSecond-Order Reaction Kinetics Overview
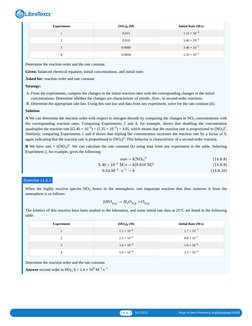
This document discusses second-order reactions, which have rates proportional to either the square of one reactant's concentration or the product of two reactants' concentrations. It provides the differential and integrated rate laws for a simple second-order reaction where 2A yields products. The document uses experimental data to show that the dimerization of a monomeric compound follows second-order kinetics, with a rate constant of 4.1 M-1·min-1 determined graphically from a plot of 1/[A] vs. time.
Uploaded by
D.T.ACopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views5 pagesSecond-Order Reaction Kinetics Overview
This document discusses second-order reactions, which have rates proportional to either the square of one reactant's concentration or the product of two reactants' concentrations. It provides the differential and integrated rate laws for a simple second-order reaction where 2A yields products. The document uses experimental data to show that the dimerization of a monomeric compound follows second-order kinetics, with a rate constant of 4.1 M-1·min-1 determined graphically from a plot of 1/[A] vs. time.
Uploaded by
D.T.ACopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd