0% found this document useful (0 votes)
168 views14 pagesCholinergic Pharmacology Overview
The document discusses cholinergic pharmacology, including:
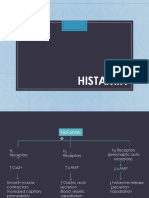
1. The synthesis, storage, and release of acetylcholine (ACh) in cholinergic neurons and its degradation by acetylcholinesterase.
2. The types of muscarinic and nicotinic cholinergic receptors that ACh acts on in the central and peripheral nervous systems.
3. The classification of cholinomimetic drugs including direct-acting choline esters and alkaloids as well as indirect-acting choline esterase inhibitors.
Uploaded by
Ali EllaffiCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
168 views14 pagesCholinergic Pharmacology Overview
The document discusses cholinergic pharmacology, including:
1. The synthesis, storage, and release of acetylcholine (ACh) in cholinergic neurons and its degradation by acetylcholinesterase.
2. The types of muscarinic and nicotinic cholinergic receptors that ACh acts on in the central and peripheral nervous systems.
3. The classification of cholinomimetic drugs including direct-acting choline esters and alkaloids as well as indirect-acting choline esterase inhibitors.
Uploaded by
Ali EllaffiCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd