85%(13)85% found this document useful (13 votes) 42K views336 pages3rd Edition Complete Chemistry For Cambridge IGCSE
Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content,
claim it here.
Available Formats
Download as PDF or read online on Scribd
(@) OXFORD
CMe Tas tT
Chemistry
ie) g
Bicol)
yas et
Gere Merl eters es
U1 Tie 1 ee
a�OXFORD
oes ein be Loco Yona tino
Soo en a ae
eee ee
Hosmer han a oeprish peseatiy nn ersekeiecagoclen ae
Seeioene ceeese eee
fee eee,
ent psn oi bk a seep de ied
Fopuion tm come afore en
Acmontgets
‘Seca rin herd anor Cane ered amos
‘piesa ami et tat nk
2 Ras gens 2 Suber: Dan Gaiety
Eogntnat obec cig hese fete ner has,
‘alt ips Mu try anc pa Tatoos)
Shaeroo fo Sex uoasameac po Sats poe
Brsertag yer tance i Crepe
eee eevee eeeneeate
Scenes cerca prereata
Sean. eo eemente
See meters eee
one erent meee
Se eee tee
ee ee
Beer mes acielerrcerens
ees ences inet cen
tester giclee et bsp Shey eee aa
Evcar haber cecmiatiaermutamer arty
Ee cere pater
Sere eee ere
Bebe cin eter et wean
rebyShmere pie OF pas ueskashutcrane pe GU ro shnchtock NS
So es ee
Ses See eer rene
So eee
Eber rete Neds fe alts Ragan po Pat
Sco een ar ay
See eae aein eieecnnenet nae
Sot
epoca eater reat
Essa ereriac cases ate
Sa etre eee
Reet aires
eas aateae ge oman cient ot ea
epee an nema ae
Eeorrtetes ie me eeeceecee mega
Bane petite eerie aarroeae Pe
Sees see a eg
seattle ear ce Ger ly
Hin mare ete ee
eshcrereeerna ciate
Ca
Fraime Naeem cee
Epes a meer
Se acetone een,
Soe eee ees
Sep eee toe
cal naif cat eee
Seca naearee emetereer eer
Seetickets a
Fegan” dee ceo Seat”
Baier eden s aenrecn er tara
es tent eer
Sees cme reer
Boesteeaetnicir meaner aa
oe ener ene
‘hc ple sy Me arsenite pie Opes bere
ftraneesectamio Cif Cnc 19 4972\Pomt Colla © Boar tendon
ee ey eee emi
ey cidieeriar eta
a a ae a
Some Seren ioaet peur pare
Socata neemneen tees
Seater re rerencreaee a
Seer enna,
nee een er ereninanane
ae eee eee
cence eatreceeeat ter ea
Beri afeanauienee ae naan fe
See ee ee ea ren
Eaperarencen tector
Sienna ame
‘Suter fami Sta ncn. pb Mp Maroc
Scie sects reat an
‘Sy ogee haa po
Seater shiner amennemras
Scheme, ena pt
Stes rmeeermiereetmcnny
See aa Cerne
Scanner ater aar meer,
2 eee eee eer
Soc cei sae
See oe enercters eect
re ape Roe eee
Seer,
Seine pe ates ene
Shama aemameheae a
See ee
Soe
Sees a eeciemie matey
See
ae
Soaeng emer nemmecies
See era eee
eaten peta et are oe
nee elytra
Sees ee re
ore enorme toes
Si caotareenor arerecnom ea
Severin ar fa
ciate een aera
Speers eee etn
Se arma coer
iporaihee isn ert a
Se ack eear eer
Genre ee ne
epee nk eer ee ara
Epes aethtta ae atta pe rr
{te PhtogrciyScen Fico Liven it: aaah, GOOF
SEY EIopaRe ata es Packs taaaaterec ps Ace
[Enter Psorapiysionse te iba
‘dle nero enti i epi fr te iil ane
tegen i em hse pesca nae th pe�Introduction
If you are taking IGCSE chemistry, using the Cambridge International
Examinations syllabus 0620, then this book is for you. It covers the
syllabus fully
Finding your way around the book
The contents list on the next page shows how the book is organised.
Take a look, Note the extra material at the back of the book too: for
example the
juestions from past exam papers, and the glossary.
Finding your way around the chapters
Each chapter is divided into two-page units. Some colour coding is used
within the units, to help you use them properly
Core syllabus content Extended syllabus content
Ifyou are Following the Core For this, you need aff the
syllabus content you can ignore material on the white pages,
any material with a red line including the material marked
beside it with a red line
Extra material Chapter checkups
.e8 of this colour contain ‘There isa revision checklist
extra material for some topics, at the end of each chapter,
We hope that you will ind it and also a set of exam-level
interesting ~ but itis not ‘questions about the chapter
needed for the exam on a coloured background,
Making the most of the book and support website
We want you to understand chemistry, and do well in your exams.
This book, and the website, can help you, So make the most of them!
Work through the units The (vo-page units will help you build up
your knowledge and understanding of the chemistry on your syllabus,
Use the glossary If you come across a chemical term that you do not
understand, try the glossary. You can also use the glossary to test yourself
Answer the quest
ns [Cis great way to get to grips with a topic
This book has lots of questions: at the end of each unit and
ich chapter,
and questions from past exam papers at the end of the book.
Answers to all questions, exce
those from exam papers, are at the back
of the book, Your teacher can provide answers for the
Use the website The website has an interactive test for each chapter
advice on revision, exam-style questions, and more.
And
We hope this book will help you to enjoy it, and succeed in your course
nally, enjoy! Chemistry is an important and exciting subie
RoseMarie Gallagher
Pauil Ingram�Y Contents
Cerne
Eventing is made of pateles 2 “The mote n
1.2 Solids, ques and gases 4 6.2 Calculations rom equations using themole 74
113° Theparticesin sos, guts andgases =< ~—=—.3._eations involving gases %6
1A Acoser look at gases 8 GA. The concentration of soliton 78
Checkup on Chapters 106.5. Findingtheempincal forma 0
6.6 From empirical to inal formuta 2
6.7 Finding % yes and % purty a
2.1 Mixtures, solutions, and solvents 12 Checkup on Chapter 6 86
2.2 Pure substances and impurities 14
2.3. Separation methods (part) 16
244 Separation methods (part) 1 7 oxidation nd reduction 38
2.5. Mere about paper chromatography 207.2. Redoxand electron transfer 90
The chromatography detectives 2 7.3. Redanand changes n oxidation state 2
Checkup on Chapter2 24 7.4. Oxidising and reducing agents 98
Checlup on Chapter7 36
3.1 Atoms and elements 26 GO Electricity anc chemical change
3.2 Nore aout atoms 28 FL conductors and insulators 98
33 Isotopes andradicactity 30.2. Theprincinles of eectrlyis 100
3.4 Howelectrone are sanged 528.3. Thereactions atthe electrodes 102
How our model of te atom developed 348.4 Theelectrolsis of brine 10a
The atomsthe inside story 368.5 Twomoreuresoelecroysis 106
3.5. Themetals and non-metats 28 Checkup on Chapter 8 108
Checkup on Chapters 40
ph S11 Energy changes in reactions 110
Compounds, mixtures, and chemicalchange 42 -—=«9.2._Explaning energy changes m2
\why do atoms form bonds? 44913 Energy fom els na
The one bond 45-914 Giving ou enersyaslectrcty 16
More about ons “8 “The batteries in your fe ne
The covalent bond 509.8 Reversibereactions 120
Comtent compounds 52 8.8. Shing the equa 122
Comparing onic and covalent compounds 54 Checkup on Chapters na
Giant covmlent structures 6
The bonding in metals 58
Checkup on Chapters 60 TO. Ratesofreaction 1%
202. Messurng the rate ofa eaction 128
ee 103 Changing the rate of a reaction (part 1) 130
‘The names and formule of compounds 104 Changing therate ofa reaction (parm) 132
Equations for chemical reactions 64 105 Explaining ates 4
Themasses of atoms, molecules, andions «65S «108. Catalysts 136
Some calculations about masses and% 68 Nore about enzymes 8
Checkup on Ghaplers 70407. Photochemical reactions 0
Checkup an Chapter 10 142�162 Making ammonia in industry 22
183. Fertilisers 228
164 Sulfurand sulfur dioxide 226
165 Sulfuric acid 228
11 Carbon and the carton cycle 230
16.7 Some carbon compounds 232
168 Greenhouse gases, and labal warming 234
18.9 Limestone Ras
Checkup on Chapter 16
Petroleum: a fossil fuel
Refining petroleum ae
a3 Cracking hydrocarbons 24a
117A Families of organic compounds 246
175 Thealkanes 248,
176 Thealkenes 250
177 Thealcohols 252
178 The carboxylic acids a
(Checkup on Chapter 17
Q Introducing polymers
18.2 Addition polymerisation 260
1B3 Condensation polymerisation 262
14 Making use of synthetic polymers 264
BS Plastics: here to stay? 266
186 Natural polymers in food (part 1) 268
18.7 Natural polymers in food (part II) 270
Checkup on Chapter 18
Pr
)
XH chem: a racteat aie
Gel Somunatoneinat me
Sa any apne a se
aed. Teter ieee ators ie
Tham unto apes 202
tan seston ben baoe aa
closy 220
Taninminnwmcwame an
Support website: www [Link]/9780198399148�ED Everything is made of particles
Particles everywhere
Rock, air, and water look very different, But they have one thing in
common: they are made of tiny pieces. Let's call these pieces particles,
teverythi
around you is made of particles ~ and so are you!
In rock and other solids, the particles are not free to move. But in liquids
and gases, they are always moving ~ and colliding with each other
Proof from pollen
For centuries, people had guessed that water and ar were made of tiny
particles. But since they could not see them, they could not prove it
Then in 1827, a botanist called Robert Brown noticed something strange.
He was studying pollen from a flower, in water, under a microscope
He siw particles froin the pollen jigeting around. They were not alive —
78 years later, Albert Einstein came up with the answer The particles
moved because they were being struck by tiny invisible moving water ‘A. All made of pattces!
particles. In fact their movement proved that water is made of particles
Brownian motion
Today the random motion of particles that you ean see with the naked
eve, or under'a microscope, is called Brownian motion, The particles
follow a zig-zag path, because they are being struck by tiny invisible
particles. Look at the drawing on the right.
on
More evidence me
Everyone now accepts that things are made of particles. Most are far too The random motion of a parte.
small to be seen under a microscope. But there is plenty of evidence for This is what Robert Brow observed.
them. Let's look at more examples,
Outside the tab
at
1. In sunlit rooms, you sometimes see dust dancing 2. Cooking smells spread. The ‘smells’ are due to tiny
in the air. It dances because the dust particles are being particles, which spread because they are bombarded
bombarded by the tiny moving particles in ait: This is _by the particles in air: This is an example of diffusion,
an example of Brownian motion. (See next page.) Some of them end up in your nose!�Inthe lab
parle toa
pails om
thecal mix
sang the
‘water particles a
theeystal
3. Place a crystal of purple potassium manganate(VI1)
ina beaker of water. The colour spreads through the
water, because the particles leave the solid crystal ~ it
dissolves — and spread among the water particles.
Diffusion
Look again at the two examples above. The particles mix and spread by
colliding with other moving particles, and bouncing off in all directions,
‘This mixing process is called diffusion.
bron
‘The end result is that the particles spread from where they are more
concentrated to where they are less concentrated, until they are evenly
mixed. Look at the drawings on the right.
‘So what are these particles?
In examples 2, 3, and 4, we cannot see the moving particles ~ even with
the most powerful microscope, They are far too small. So what are they?
‘© The smallest particles, that we cannot not break down further in
chemical reactions, are called atoms.
© In some substances, the particles are just single atoms, For example
air contains single argon atoms.
© In many substances, the particles consist of two or more atoms joined
together: These particles are called molecules. Water and bromine
exist as molecules, Air is mostly nitrogen and oxygen molecules,
© In other substances the particles are atoms or groups of atoms that
carry a charge. These particles are called ions. In example 3 above,
the particles in potassium manganate(VII) are ions,
You'l find out more about all these particles in Chapters 2 and 3.
But what about the particles Robert Brown observed? They contained
thousands of atoms. That's why they were big enough to be seen with
a microscope. Dust particles in air contain thousands of atoms too.
panicle
bromine parties
and ar partes
row fly mined
bromine
ponte
4 Place an open gas jar of air upside down on an open,
sas jar containing a few drops of red-brown bromine,
The colour spreads upwards because particles of
vapour mix among the gas particles in the air,
‘The moving parices colide, en
bounce apart inal directions
eo
°
e
eo %e 3
e
@%e0"o
ce « ©" a
oe 2
e
eo" e%o
Inia ey area ed
{But they keep on moves)�STATES OF MA
rE? Solids, liquids, and gases
What's the difference?
It is easy to tell the difference between a solid, a liquid, and a gas:
solid has a fixed shape and a fixed A liquid flows easily. It has a fixed
volume. It does not flow. Think of volume, but its shape changes. I.
all the solid things around yous their takes the shape of the container
shapes and volumes donot change. you pour it into,
Water: solid, liquid and gas
Water can be a solid (ce), liquid (water), and a gas (water vapour
am) Ts state can be changed by heating oF co
thermometer
shows Le water vancur
ice cubes meting
1 Ice slowly changes to water, 2) When the water is heated its
when itis put in a warm place. temperature rises, and some of it
This change is called melting, changes to water vapour. This
The thermometer shows 0°C until change is called evaporation,
all the ice has melted. So 0°C is ‘The hotter the water gets, the
called iis melting point. more quick!
it evaporates,
And when steam is cooled, the opposite changes take place:
A gas does not have a fixed volume
fr shape. It spreads out to fil its
container: Itis much lighter than
the same volume of solid or liquid,
— thermometer
‘hows 100°C
(isle)
isle
boing weer
eat
3 Soon bubbles appear in the
‘water: [tis boiling, The water
vapour shows up as steam.
‘The thermometer stays at 100°C
while the water boils off. 100°C is
the boiling point of water
sear condenses to fom nater
coo! below 100° coolbelow 0% freeze or soliifies
‘o fom ce
You can see that
© condensing is the opposite of evaporating
© freezing is the opposite of melting
© the freezing point of water is the same as the melting point of ive, 0°C.�ewarTER
Other things can change state too ¥ olten iron being poured out at an
It’s not just water! Neatly all substances can exist as solid, liquid, and gas. iran works. Hot ~ over 1540°C:
Even iron and diamond can melt and boil! Some melting and boiling
points are given below. Look how different they are.
coygen 219
ethanal aE
sodium i 8
sulfur 19
‘ron i 1540 |
diamond 3550 4332
Showing changes of state on a graph
Look at the graph on the right below. It shows how the temperature
changes as a block of ice is steadily heated. First the ice melts to water.
Then the water gets warmer and warmer, and eventually turns to steam,
‘This (ype of graph is called a heating curve, esting cure for weiter
Look at the step where the ice is melting. 150
vapou
Once melting starts, the temperature stays
at 0°C until aif the ice has melted " ‘pater baling fas
Then when the water starts to boil, the
temperature stays at 100°C until all the
water has turned to steam. — |
ind boiling points are tvapcsston ofc |
°
‘You ean draw a heating curve for any os |
substance. -o tell
‘Sublimatior
‘Some substances go straight from solid to
special change
when they warm up.
This change is called sublimation,
For example, if you leave solid (frozen) carbon dioxide sitting at room
temperature, it will sul And if you warm some iodine crystals
gently on a clock glass, they will give off a purple vapour
Naphthalene, which is used in old-fashioned moth balls, also sublimes.
Reapers Rltanse ‘4. Carbon diaxide sublining. Solid
carton dioxide fs also called ey ice.�TATES OF MAT
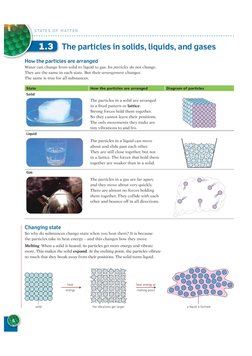
VED The particles in solids, liquids, and gases
How the particles are arranged
Water can change from solid to liquid to gas. Its particles do not change.
They are the same in each state, But their arrangement changes,
The same is true for all substances.
‘The particles in a solid are arranged
ina fixed pattern or lattice.
Strong forces hold them together:
So they cannot leave their positions.
‘The only movements they make are
tiny vibrations to and fro,
‘The particles in a liquid can move
about and slide past each other:
They are still close together, but not
in a lattice. The forces that hold them
together are weaker than in a solid,
Gas
The particles in a gas are far apart,
and they move about very quickly.
‘There are almost no forces holding
them together: They collide with each
other and bounce off in all directions,
Changing state
So why do substances change state
Melting When a solid is heated, its particles get more eng
more. This makes the solid expand. At the melting point, the particles vibrate
so much that they break away from their positions. The solid turns liquid,
when you heat them? It is because
the particles take in heat energy and this changes how they move.
and vibrate
ee
reking point
‘he vibrations ge larger
a liquids formed