0% found this document useful (0 votes)
439 views1 pageSRL Format 2
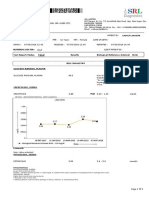
This diagnostic report provides the results of urinary protein creatinine ratio testing for a patient named Dummy with a patient ID and date of birth. The test was drawn on July 30, 2021 and reported on July 31, 2021. The results showed protein in the urine was less than 11.9 mg/dL, creatinine in the urine was not established, and the protein/creatinine ratio was not established. The report also provides conditions of laboratory testing and reporting.
Uploaded by
haroon012023Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
439 views1 pageSRL Format 2
This diagnostic report provides the results of urinary protein creatinine ratio testing for a patient named Dummy with a patient ID and date of birth. The test was drawn on July 30, 2021 and reported on July 31, 2021. The results showed protein in the urine was less than 11.9 mg/dL, creatinine in the urine was not established, and the protein/creatinine ratio was not established. The report also provides conditions of laboratory testing and reporting.
Uploaded by
haroon012023Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd