0% found this document useful (0 votes)
861 views1 pageHalide Ion Tests with Silver Nitrate
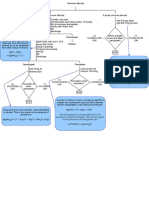
This document describes tests to identify halide ions using silver nitrate and concentrated hydrochloric acid.
1. Silver nitrate is added to produce characteristic precipitates with chloride (white AgCl), bromide (creamy AgBr), and iodide (creamy-yellow AgI) ions.
2. Ammonia is used to confirm the identity of the ions by dissolving certain precipitates: dilute ammonia dissolves AgCl and concentrated ammonia dissolves AgBr, but AgI does not dissolve in ammonia.
3. Concentrated hydrochloric acid produces characteristic reactions and observations with each halide ion to identify it, such as producing white fumes of
Uploaded by
Tilak K CCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
861 views1 pageHalide Ion Tests with Silver Nitrate
This document describes tests to identify halide ions using silver nitrate and concentrated hydrochloric acid.
1. Silver nitrate is added to produce characteristic precipitates with chloride (white AgCl), bromide (creamy AgBr), and iodide (creamy-yellow AgI) ions.
2. Ammonia is used to confirm the identity of the ions by dissolving certain precipitates: dilute ammonia dissolves AgCl and concentrated ammonia dissolves AgBr, but AgI does not dissolve in ammonia.
3. Concentrated hydrochloric acid produces characteristic reactions and observations with each halide ion to identify it, such as producing white fumes of
Uploaded by
Tilak K CCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
/ 1