Professional Documents
Culture Documents
Silica
Uploaded by
Memo Yassin0 ratings0% found this document useful (0 votes)
53 views9 pagesIn atmospheric pressure silica exist in 3 forms ; quartz(till 870o), 870o tridymite(870o-1470o) and cristobalite(1470o-1710o) Each form has two structure in high temp. ( form) and low temp.( form) there are other forms of silica in higher pressure such as; coesite, keatite and stishovite.
Original Description:
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentIn atmospheric pressure silica exist in 3 forms ; quartz(till 870o), 870o tridymite(870o-1470o) and cristobalite(1470o-1710o) Each form has two structure in high temp. ( form) and low temp.( form) there are other forms of silica in higher pressure such as; coesite, keatite and stishovite.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
53 views9 pagesSilica
Uploaded by
Memo YassinIn atmospheric pressure silica exist in 3 forms ; quartz(till 870o), 870o tridymite(870o-1470o) and cristobalite(1470o-1710o) Each form has two structure in high temp. ( form) and low temp.( form) there are other forms of silica in higher pressure such as; coesite, keatite and stishovite.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 9
INTRODUCTION
In atmospheric pressure silica
exist in 3 forms ;
quartz(till 870º) ,
tridymite(870º-1470º) and
.cristobalite(1470º-1710º)
Each form has two structure
in high temp.(αform) and
low temp.(βform)
Quartz has less open
structure than the other
two which can be seen in
their densities;
quartz=(2.6g/cm³) ,
tridymite=(2.3g/cm³) and
crisobalite=(2.27g/cm³)
INTRODUCTION
There are other forms of
silica in higher
pressure such as;
coesite, keatite and
.stishovite
β-quartz can be seen as
helices either left or
right handed , due to
its optical activity it is
either laevo or dextro
.rotary
CLASSIFICATION
Silicates are classified into 6 categories
according to the way of linkage between
.the tetrahedral silica units
: Those categories are
.Orthosilicates-1
.Pyrosilicates-2
.Cyclic silicates-3
.Chain silicates-4
Layer silicates-5
.Framework silicates-6
Orthosilicates-1
Contain [(SiO4)2-]
ions , metal ions
occupy interspaces
surrounded by
oxygen atoms
according to their
.size
For example: olivin
.and zircon
Pyrosilicates-2
The variation of (Si-
O-Si) bond length
is the main interest
.of this type
The most common
example is the
Melitite family such
.as baysilite
Cyclicsilicates-3
In this type ions are
arranged as layers
but with the metal
ions lying between
these layers and
bind them
.together
.For example; beryl
Chainsilicates-4
groups share 2 vertices in)MX4(
this type to show (MX3)
.structure for single chain
For single chain there are
pyroxene group
.(spodumene)
As for double chains part of
the tetrahedrals share2
vertices and the rest share
3 vertices. Amphipoles are
a group of minerals with
double chain structure
.(termolite)
Layersilicates-5
.Tetrahedral units sharing 3 vertices
There are 3 classes for this type;
(i)single layer: forming different
types of nets
(6net,4:8net,4:6:8net,4:6:12net)
Simple buckled layer: sharing 3 vertices)ii(
., 4vertices or both
complecated buckled layer: unshared)iii(
vertices of two layers pointing to the
same side can combine to form a
.double layer with all vertices shared
Then it can be classified to ;(a)
type1(chrysolite),(b)
type2(pyrophyllite),(c)charged
type2(micas),(d) charged type2
with hydrated ions (clays), (e)
.charged of both types
Frameworksilicates-6
The possible structures are: (a)3
vertices shared (b)3or4 vertices
.shared (c)4 vertices shared
; There are three groups of this type
Feldspars:-discribed as space-)1(
filling arrangement of polyhedral.
These are classified to
:aurthoclase (aurthoclase,
celsian) and plagioclase (albeit)
Zeolites:- have more open)2(
structure and take up water very
easy. Those have 3types: fibrous
.& lamellar& edges zeolites
Ultramarine:- colored materials)3(
used as pigments include lapis
.lazuli mineral . Such as sodalite
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- 07 Ravat GPEncyPart3 ReductionToPoleDocument3 pages07 Ravat GPEncyPart3 ReductionToPoleRosyad NajdanNo ratings yet
- ASEM 2021 Conference PaperDocument6 pagesASEM 2021 Conference Paperneil beeraspatNo ratings yet
- Geophysical and Geochemical Exploration TechniquesDocument19 pagesGeophysical and Geochemical Exploration TechniquesAshrafNo ratings yet
- Construction: William H. GrantDocument9 pagesConstruction: William H. GrantJhulinho Ccallo zuñigaNo ratings yet
- Cluster University of Jammu: (Established by Govt. of J&K) CBCS Exam Scheme Marks-Cum-Grade-SheetDocument2 pagesCluster University of Jammu: (Established by Govt. of J&K) CBCS Exam Scheme Marks-Cum-Grade-SheetIbrar AhmedNo ratings yet
- JPT 2017-OctubreDocument116 pagesJPT 2017-Octubreabraham dominguezNo ratings yet
- And of Clay Are We Created SummaryDocument3 pagesAnd of Clay Are We Created SummaryKinn De Villa-Ares100% (1)
- Earth and Life Science: Quarter 1 - Module 2 (Senior High School)Document15 pagesEarth and Life Science: Quarter 1 - Module 2 (Senior High School)JAY ELLNo ratings yet
- Assignment 02Document12 pagesAssignment 02Syeda L RNo ratings yet
- Geography Chapter 2Document14 pagesGeography Chapter 2Ananya RawatNo ratings yet
- Rock Bolt SpecisDocument1 pageRock Bolt SpecisZain AbidiNo ratings yet
- The Geochemistry of Gems and Its Relevance To Gemology: Different Traces, Different PricesDocument4 pagesThe Geochemistry of Gems and Its Relevance To Gemology: Different Traces, Different PricesjaredNo ratings yet
- A Review of Methods For Measuring Groundwater-Surfacewater Exchange in Braided RiversDocument21 pagesA Review of Methods For Measuring Groundwater-Surfacewater Exchange in Braided RiversLady Johana Buritica CortesNo ratings yet
- Hardenbol Et Al., SEPM Charts, 1998Document8 pagesHardenbol Et Al., SEPM Charts, 1998Shifeleni LPNo ratings yet
- Curriculum Vitor Vieira Vasconcelos - December - 2017Document3 pagesCurriculum Vitor Vieira Vasconcelos - December - 2017Vitor Vieira VasconcelosNo ratings yet
- Spectral and Amplification Characteristics in San Salvador City (El Salvador) For Upper-Crustal and Subduction EarthquakesDocument9 pagesSpectral and Amplification Characteristics in San Salvador City (El Salvador) For Upper-Crustal and Subduction EarthquakesSergio Ito SunleyNo ratings yet
- Kuliah PSB 02 NewDocument10 pagesKuliah PSB 02 NewadhityamspNo ratings yet
- Performance and Cutter Life Assessments in Hardrock TunnellingDocument7 pagesPerformance and Cutter Life Assessments in Hardrock TunnellingGeo Khader AbdulNo ratings yet
- 12 Perez Ramon TecsolGeo DelftDocument29 pages12 Perez Ramon TecsolGeo DelftahmedNo ratings yet
- 01 Muskan 12th CommerceDocument13 pages01 Muskan 12th Commercemuskan0% (1)
- ACTIVITY 1 Location of The Earthquake EpicenterDocument2 pagesACTIVITY 1 Location of The Earthquake EpicenterB - HERRERA, Jhian Carlo G.No ratings yet
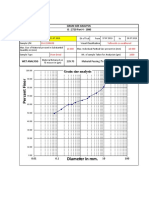
- Diameter in MM.: Grain Size AnalysisDocument13 pagesDiameter in MM.: Grain Size AnalysisImran KhanNo ratings yet
- Planning Web Victoria PecotDocument3 pagesPlanning Web Victoria Pecotapi-534405902No ratings yet
- Lesson:: Learning Activity Sheet in Science 10 First Quarter - Week 2Document4 pagesLesson:: Learning Activity Sheet in Science 10 First Quarter - Week 2Julius RaquelNo ratings yet
- PT Sci. 9Document8 pagesPT Sci. 9kathleen de jesusNo ratings yet
- Underbalance Drilling ManualDocument36 pagesUnderbalance Drilling ManualFelipe OliveiraNo ratings yet
- Harraden Whole ThesisDocument394 pagesHarraden Whole Thesisjair pieroNo ratings yet
- Gallanosa National High School San Pedro, Irosin, Sorsogon Learning Activity Sheet Number 4Document7 pagesGallanosa National High School San Pedro, Irosin, Sorsogon Learning Activity Sheet Number 4Maxine ReyesNo ratings yet
- Science 6: Buenasher Learning Academy IncDocument7 pagesScience 6: Buenasher Learning Academy IncEl CruzNo ratings yet
- Updated Ten Year Ecological Solid Waste Management PlanDocument142 pagesUpdated Ten Year Ecological Solid Waste Management PlanTrisha DamianNo ratings yet