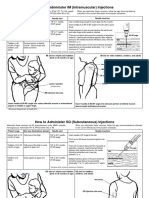
Professional Documents
Culture Documents
Eld10500 FM
Uploaded by
Scott WylieOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Eld10500 FM
Uploaded by
Scott WylieCopyright:
Available Formats
CLINICAL UPDATE
Vaccine components and constituents:
responding to consumer concerns
Barbara E Eldred, Angela J Dean, Treasure M McGuire and Allan L Nash
T he World Health Organization states “The two public health
interventions that have had the greatest impact on the world’s
health are clean water and vaccines”.1 However, rates of
vaccination uptake in Australia may be suboptimal.2,3 Concern about
vaccine safety is a potential barrier to immunisation; and reductions
ABSTRACT
• Vaccination remains a vital strategy in the prevention of
infectious disease.
• Commercial vaccine formulations contain a range of additives
in vaccination rates have been described after media reporting of or manufacturing residuals, which may contribute to patient
adverse effects.4 concerns about vaccine safety.
Vaccination safety is a frequent motivation for consumers to
The Medical Journal of Australia ISSN: 0025- • Primary health care professionals are well placed to address
contact drug information services operating out of the Mater Health patient concerns about vaccine safety. We describe the key
729X 20 February 2006 184 4 170-175
Services in Brisbane
©The Medical(personal
Journal reflection). Vaccines
of Australia 2006 contain constitu- constituents present in vaccines, discuss issues related to
ents such as preservatives,
www.mja.com.au stabilisers, adjuvants and biological safety and acceptability of these constituents, and provide
growth media,update
Clinical which may contribute to consumer concern about a table highlighting constituents of commercially available
vaccine safety; specifically: vaccines in Australia.
• presence of preservatives; MJA 2006; 184: 170–175
• likelihood of allergic reactions; and
• constituents of human or animal origin.
Community-based health professionals are in a key position to Preservatives
address these concerns by providing accurate information about
vaccine constituents and components. However, much of this infor- Thiomersal
mation is not readily available. We sought to compile a comprehen- Thiomersal (sodium ethylmercuric thiosalicylate) is an organic com-
sive overview of constituents, culture and growth media used in the pound containing ethylmercury that has been widely used as a
production of the vaccines available in Australia. vaccine preservative since the 1930s. Concerns have focused on its
potential toxicity as a heavy metal, and its role in the claimed link
Information retrieval between measles, mumps and rubella (MMR) vaccination and
Information was retrieved using the following iterative process: autism.5
• The Therapeutic Goods Administration (TGA) website was used A recent study assessed whether multiple vaccinations can lead to
to ascertain vaccines registered in Australia. mercury accumulation.6 In full-term infants exposed to vaccines
• Manufacturers were contacted to confirm current products. containing thiomersal, mercury concentrations detected in blood
• This was compared with the Australian Immunisation Handbook (range, 2.85–20.55 nmol/L) were well below the level thought to be
to ensure all products were included. associated with adverse effects. Additionally, ethylmercury appears to
• Information about excipients and media was obtained from be eliminated via the gastrointestinal tract soon after exposure
registered Product Information. (estimated half-life, 7 days).6 Because the developing fetus and low
• Where clarification of constituents was required, manufacturers birthweight babies are more vulnerable to toxic effects of mercury, it
were contacted. has been suggested that exposure to vaccines containing thiomersal
• Final table sections were submitted to individual manufacturers at time of birth may pose some risk in very low birthweight
for review. premature infants.6 To minimise any potential risk, all vaccinations in
Information is presented in two tables: childhood vaccines (Box 1) the Australian Standard Vaccination Schedule for children younger
and travel vaccines (Box 2). As vaccine manufacture is subject to than 5 years are now thiomersal-free or contain only trace amounts.7
ongoing development and change, information provided in this In 1998, a case series was published describing 12 children with
review is current at the time of preparation. pervasive development disorder with gastrointestinal features.8 The
report claimed that exposure to MMR vaccination may have been
FOR EDITORIAL COMMENT, SEE PAGE 150
linked to emergence of behavioural symptoms. Since then, contro-
Education and Information Unit, Mater Pharmacy Services, versy has surrounded the potential association between MMR vaccine
Mater Misericordiae Hospital, South Brisbane, QLD. and autism, with thiomersal a suggested culprit. The original claims
Barbara E Eldred, BPharm, Drug Information Pharmacist; have been criticised for being based on uncontrolled, anecdotal
Angela J Dean, BPharm, PhD, Research Fellow, Kids in Minds Research, associations, and more recently, some of the study authors have
Mater Child and Youth Mental Health Service; Treasure M McGuire,
BPharm, BSc, PhD, Assistant Director, Practice and Development; and retracted their interpretation associating MMR vaccines with autism.9
Senior Conjoint Lecturer, School of Pharmacy, University of Queensland; Larger epidemiological studies have also failed to demonstrate an
Allan L Nash, BPharm, Drug Information Pharmacist. association between MMR vaccination or use of thiomersal-contain-
Reprints will not be available from the authors. Correspondence: ing vaccines and autism.10
Dr T M McGuire, Education and Information Unit, Mater Pharmacy The mercury or thiosalicylate components of thiomersal may
Services, Mater Misericordiae Hospital, Raymond Terrace,
produce hypersensitivity reactions.11 Reactions are uncommon, even
South Brisbane, QLD 4101. tmcguire@mater.org.au
in those with sensitisation, and serious reactions are rare.
170 MJA • Volume 184 Number 4 • 20 February 2006
CLINICAL UPDATE
1 Childhood vaccines
Live Biological
Generic Vaccine vaccine growth media Cells Other additives
Chicken pox (Varicella) Varilrix (GSK) Y Cow, human Human cell culture Neomycin, lactose
Varivax — refrigerated Y Gelatine, cow Human and guinea Neomycin, monosodium glutamate
(MSD) pig cell culture
Diphtheria/ tetanus ADT (CSL); CDT (CSL) N Cow or pig Biological culture Thiomersal, aluminium phosphate
or horse*
Diphtheria/ tetanus/ pertussis Boostrix (10 years) (GSK) N Cow Biological culture Formaldehyde, phenoxyethanol,
aluminium phosphate, aluminium
hydroxide
Infanrix (GSK) N Cow Biological culture Formaldehyde, phenoxyethanol,
aluminium hydroxide
Tripacel (Aventis Pasteur) N — Biological culture Phenoxyethanol, aluminium
phosphate
Diphtheria/ tetanus/ pertussis/ Infanrix Hep B (GSK) N Cow Yeast (hep B), Formaldehyde, phenoxyethanol,
hepatitis B biological culture aluminium phosphate and hydroxide
Diphtheria/ pertussis/ Infanrix IPV (GSK) N Cow Monkey cell culture, Formaldehyde, neomycin,
poliomyelitis/ tetanus biological culture phenoxyethanol, aluminium
hydroxide, polymyxin B
Diphtheria/ hepatitis B/ Infanrix Penta (GSK) N Cow Yeast (hep B), Formaldehyde, neomycin,
pertussis/ poliomyelitis/ tetanus monkey cell culture, phenoxyethanol, aluminium
biological culture phosphate and hydroxide, polymyxin
B
Diphtheria/ Haemophilus Infanrix Hexa (GSK) N Cow Yeast (hep B), Formaldehyde, neomycin,
influenzae/ hepatitis B/ monkey cell culture, phenoxyethanol, lactose,
pertussis/ poliomyelitis/ tetanus biological culture aluminium phosphate and hydroxide,
polymyxin B
Diphtheria toxoid Diphtheria Vacc (Adsorbed) N Cow or pig Biological culture Thiomersal, aluminium phosphate
(CSL); Diphtheria Vacc or horse*
(Adsorbed) (Adult) (CSL)
Haemophilus influenzae type B Liquid Pedvax HIB (MSD) N — Biological culture Aluminium hydroxide
Haemophilus influenzae type B Comvax (MSD) N — Biological culture, Aluminium hydroxide
with hepatitis B yeast
Hepatitis B Engerix - B (GSK); Engerix- N Cow Yeast Thiomersal, aluminium hydroxide
B (Paediatric) (GSK)
H-B Vax II (MSD); H-B Vax II N — Yeast Formaldehyde, aluminium hydroxide,
Dialysis (MSD); H-B Vax II potassium thiocyanate
(Paed) (MSD)
Influenza Fluad (Chiron) N Chicken, egg — Formaldehyde, neomycin, squalene
(shark liver oil), kanamycin sulfate
Fluarix (GSK) N Cow, egg Hen’s egg cell Formaldehyde, thiomersal,
culture gentamicin
Fluvax (CSL) N Egg — Neomycin, polymyxin B
Influvac (Solvay) N Egg — Gentamicin
Thiomersal in batches manufactured
before February 2005
Vaxigrip (Aventis Pasteur); N Egg — Formaldehyde, neomycin
Vaxigrip Junior (Aventis
Pasteur)
Measles/ mumps/ rubella M-M-R II (MSD) Y Gelatine, Human cell culture, Neomycin, human serum albumin
cow chick embryo cell
culture
Priorix (GSK) Y Cow, egg — Neomycin, lactose
Meningococcal C Meningitec (Wyeth) N Cow (milk) Biological culture, Aluminium phosphate, diphtheria
yeast conjugate
Menjugate (CSL) N Cow Biological culture Aluminium hydroxide, diphtheria
conjugate
NeisVac-C (Baxter) N Cow Biological culture Aluminium hydroxide, tetanus
conjugate
Pneumococcal Pneumovax 23 (MSD) N Rabbit Biological culture Phenol
Prevenar (Wyeth) N Cow (milk) Biological culture, Aluminium phosphate, diphtheria
yeast conjugate
Poliomyelitis Ipol (Aventis Pasteur) N Cow Monkey cell culture Formaldehyde, neomycin,
streptomycin, polymixin B
Polio Sabin (GSK) Y Cow Human cell culture Neomycin, polymixin B
Rubella Meruvax II (MSD) Y Gelatine, cow Human cell culture Neomycin, human serum albumin
Tetanus Tet-Tox (CSL) N Cow or pig Biological culture Thiomersal, aluminium phosphate
or horse*
* These vaccines may contain serum from any one of these sources. This differs from batch to batch and the manufacturer would need to be contacted for information about a specific
batch. Biological culture = culture of unspecified source or source may vary between batches. GSK = GlaxoSmithKline. MSD = Merck, Sharp and Dohme. Y = yes. N = no. ◆
MJA • Volume 184 Number 4 • 20 February 2006 171
CLINICAL UPDATE
2 Travel vaccines
Live Biological
Generic Vaccine vaccine growth media Cells Other additives
Cholera (Vibrio Dukoral (Aventis Pasteur) N — Biological culture Formaldehyde
cholerae)
Hepatitis A Avaxim (Aventis Pasteur); Havrix N Cow Human cell Formaldehyde, neomycin,
Junior (GSK); Havrix 1440 (GSK) culture phenoxyethanol, aluminium hydroxide
VAQTA Adult (MSD); VAQTA N Cow Human cell Formaldehyde, aluminium hydroxide
Paediatric/Adolescent (MSD) culture
Hepatitis A and Twinrix 720/20 (GSK); Twinrix Junior N Cow Human cell Formaldehyde, neomycin,
hepatitis B 360/10 (GSK) culture, yeast phenoxyethanol, aluminium
(hep B) phosphate and hydroxide
Hepatitis A and Vivaxim (Aventis Pasteur) N Cow Human cell Formaldehyde, neomycin,
Salmonella typhi culture phenoxyethanol, aluminium hydroxide
Japanese Je-Vax (Aventis Pasteur) N Gelatin Live mice Formaldehyde, thiomersal,
encephalitis monosodium glutamate
Meningococcal Menomune (Aventis Pasteur) N Cow Biological culture Lactose
Mencevax ACWY (GSK) N Cow Biological culture Lactose, phenol
Plague Plague Vaccine (CSL) N Cow, pig Biological culture Phenol
(Yersinia pestis) or horse
Q fever Q-Vax (CSL) N Egg — Formaldehyde, thiomersal
(Coxiella bunetii)
Rabies Mérieux Inactivated Rabies Vaccine N Cow Human cell Neomycin, β-propiolactone, human
(Aventis Pasteur) culture serum albumin
Tuberculosis BCG Vaccine (Aventis Pasteur) Y
Typhoid Typhim VI (Aventis Pasteur); Typherix N Cow Biological culture Phenol
(Salmonella typhi) (GSK)
Typhoid oral Typh-Vax Oral (CSL) (discontinued) Y Gelatin Biological culture Lactose
(Salmonella typhi)
Yellow fever Stamaril (Aventis Pasteur) Y Cow, egg — Lactose
Biological culture = culture of unspecified source or source may vary between batches. GSK = GlaxoSmithKline. MSD = Merck, Sharp and Dohme. Y = yes. N = no. ◆
Phenoxyethanol diet or medications such as antacids,7 and is well below the current
2-Phenoxyethanol is an alternative to thiomersal. One report minimum risk level of 2.0 mg/kg per day.16
describes generalised eczema occurring after vaccination where 2- The presence of aluminium adjuvants has been associated with
phenoxyethanol was found to be the sensitising agent.12 injection-site reactions such as nodules, granulomas and ery-
thema.17,18 A systematic review of controlled safety studies
Antibiotics reported that vaccines containing aluminium produce more ery-
Antibiotics such as neomycin and polymyxin B are often used to thema and induration than other vaccines in young children (up to
prevent bacterial contamination during vaccine manufacture;5 they 18 months of age), and greater local pain in older children (10–18
years).19 No association was found between aluminium and more
may contribute to systemic allergic reactions, including anaphylaxis,
serious or long-term adverse effects.
or local skin reactions.13 Previous skin reactions to neomycin are not
It has been proposed that use of aluminium adjuvants may lead
considered a risk factor for anaphylaxis and are not a contraindica-
to the syndrome macrophagic myofascitis, a histological finding
tion for use of neomycin-containing vaccines.13 Neomycin concen-
where aluminium-containing macrophages infiltrate muscle tissue,
trations may vary between vaccines, but most contain only residual
and may be accompanied by a clinical syndrome of myalgia,
amounts.14
arthralgia and fatigue.20 There are about 100 published cases of
this syndrome, most from France. As no controlled studies of this
Aluminium potential adverse effect have been conducted, a causal relationship
Adjuvants are used in vaccine manufacture to enhance immune with use of aluminium containing vaccine has not been demon-
responses to the vaccine antigen. Aluminium hydroxide and strated.20
phosphate are commonly used adjuvants, and can augment type 2
(antibody-mediated) immune responses without influencing type 1 Biological growth media, additives and cells
(cell-mediated) immune responses, or cytotoxic T cell responses.15
Aluminium adjuvants also induce antigen-specific IgE responses, Gelatin
which may predispose vulnerable individuals to allergic reactions. Gelatin is a partially hydrolysed collagen, usually of bovine or
However, no data were identified associating use of aluminium porcine origin, and is one of many types of stabilisers added to
adjuvants with increased risk of allergy-related events. vaccines.5 It may be responsible for some allergic reactions
Because higher dose exposure to aluminium salts can be toxic, occurring after vaccination, with symptoms including urticaria,
guidelines have been developed for safe levels of exposure. anaphylaxis, or local reactions.21 “Egg allergies” reported after
Exposure to aluminium from vaccines is lower than intake from MMR vaccination were found to occur in individuals with no
172 MJA • Volume 184 Number 4 • 20 February 2006
CLINICAL UPDATE
sensitivity to egg,5 and were later found to represent gelatin vaccines undergo purification processes to remove cellular residu-
sensitivity. However, the incidence of anaphylaxis to gelatin is low als and have maximum limits placed on their presence.14
(about one case per 2 million doses).5
Concern regarding use of human cell lines
Chick embryos and eggs Certain cell lines (human diploid cell lines WI-38 and MRC-5)
Egg-related allergy is common, particularly in children with were derived from embryonic tissue from three elective, medically
asthma or general allergies, and may be as high as 40% in children indicated abortions conducted in the 1960s.25 This has raised
with moderate to severe atopic dermatitis.21 The risk of egg-related concerns, with reports of some individuals with religious objec-
allergy after vaccination depends on the presence of egg protein in tions to abortion refusing vaccination because they feel this makes
the final product. For example, influenza vaccine is manufactured them complicit with the original act of abortion.26
using the extra-embryonic fluids of chick embryos and contains Patients should be aware that cell lines are self-sustaining and
measurable quantities of egg proteins.21 Although the National are not the end therapeutic product; thus additional abortions are
Health and Medical Research Council (NHMRC) immunisation not needed to sustain vaccine manufacture.25,26 No human cells
guidelines state that “individuals with anaphylactic hypersensitiv- are actually present in the vaccine, and no abortions are conducted
ity to eggs should not be given influenza vaccine”,7 little evidence specifically for the purpose of harvesting cell lines. Ethicists at the
is available to identify the actual risk of serious allergic reactions in US National Catholic Bioethics Center have concluded that the
this context. In fact, one study reported that graded dosing of association between certain vaccines and abortion was non-
influenza vaccine in 27 patients with a history of anaphylactic complicit, and thus use of these vaccines is not contrary to a
reactions to egg ingestion was well tolerated and not associated
religious opposition to abortion.27
with significant allergic reactions.22 Current guidelines and evi-
dence suggest that patients with egg allergies, other than anaphyl-
axis, may be vaccinated safely.7,22,23 Religious and philosophical concerns regarding use of
MMR vaccine is developed using chick embryo fibroblast tissue, animal products
and the final commercial vaccine does not contain egg proteins. Some religious groups who have dietary restrictions for certain
Egg allergy is not a contraindication;7 previous reports of allergy- animal products may be concerned about their presence in
related adverse events occurring after MMR vaccination have now vaccines. Ingestion of pork products is forbidden in Islam, and
been attributed to the presence of gelatin.5 some Muslims may avoid medications that contain pork-derived
products.28 However, Shariah law includes the principle of “trans-
Animal and human sera formation”, where objects can be changed into another object with
Vaccine manufacture requires the production of organisms culti- totally different properties and character, and this can turn unclean
vated on an appropriate culture medium. A medium must provide objects into clean and permissible objects. Within such a ruling,
nutrients such as proteins, albumin, polypeptides, and growth gelatin made from an unclean animal may be clean and permissible
factors specific to the needs of the microbe. Animal sera are to ingest.29
frequently added to culture media to provide nutrients for micro- Jews and Seventh-day Adventists also consider pork to be
bial growth. Bovine serum is primarily used, although serum from unclean. However, Jewish law permits use of porcine-derived
pigs, horses, rabbits or humans may also be used.24 Extensive products in non-edible forms such as parenteral formulations, or
filtering processes ensure that the final vaccine contains little, if binding agents in tablets.30 Similarly, pork-derived medical prod-
any, of the original cell material. Some media are serum-free or may ucts are not prohibited for Seventh-day Adventists, although some
be of synthetic, semi-synthetic or yeast origin. individuals may prefer to avoid such products (Dr P Harrold,
Associate Director of Adventist Health Ministries, South Pacific
Yeast Division, personal communication, May 2005). Some strict vege-
tarians or vegans may also choose to avoid animal products in
Some vaccines are manufactured using Saccharomyces cerevisiae
medications and vaccines; this is largely a personal choice and is
(baker’s yeast). There are no reports of yeast-specific IgE detected
likely to vary among individuals.
in patients following exposure to vaccines manufactured using
baker’s yeast. Currently, the risk of vaccines contributing to yeast
allergy is theoretical.5 Concern regarding risk of bovine spongiform
encephalopathy (BSE)
Cell lines Use of bovine-derived products in vaccine manufacture, such as
Cell lines refer to a specific population of cells that are maintained gelatin or bovine sera, has prompted concern about whether this
in culture for extended periods. Cells used in cell lines can poses a risk of BSE. BSE is transmitted by prions, which may lead
undergo spontaneous and unlimited replication, producing an to Creutzfeldt-Jakob disease (vCJD) in humans.
unlimited lifespan for the cells, and a cell source that is continu- A number of issues indicate that vaccination was not a contri-
ously available. This removes the need for ongoing harvesting. buting factor in the cases of vCJD in the United Kingdom. Most of
More than 5000 human and animal cell lines are available. Those these people would have been vaccinated before the emergence of
used in vaccine manufacture include two diploid cell strains of BSE in British cattle.31 Additionally, vaccines are used internation-
human origin (MRC-5 and WI-38), simian-derived continuous ally, yet the outbreak of vCJD in humans was largely restricted to
and diploid cell lines, chick embryo and chick embryo fibro- the UK. Gelatin is rated as a low-risk product. Bovine serum is not
blasts.14 Residual proteins from these cell lines may be present to a actually present in the final vaccine product, and the cells that
varying degree in the vaccines produced from them. However, contribute to the vaccine are not able to replicate prions.32
MJA • Volume 184 Number 4 • 20 February 2006 173
CLINICAL UPDATE
Despite administration of tens of millions of doses of vaccines Conclusions
manufactured using bovine-derived material, there are no reported Every decision to undertake medical interventions involves a risk–
cases of BSE transmission via human or animal vaccination. Because benefit assessment. Vaccination decisions are made more emotive
of these factors, the risk is considered theoretical.24,32-34 Australian because of the influence of the anti-vaccination lobby. Patients’
reviews have concluded that vaccines used in Australia meet high concerns about vaccine components need to be taken seriously.
safety standards and that any risk of vaccines being contaminated However, accessing accurate information about vaccine constitu-
with BSE is extremely low. Australian, European and US regulatory ents, components and their safety is difficult for the busy health
bodies have developed guidelines for use of bovine material in professional. We have sought to fill this gap. Provision of accurate,
vaccine manufacture to minimise risk of BSE transmission.24,32-34 substantiated and unemotional information enables both health
Bovine material must be sourced from countries where no professionals and the public to make informed decisions concern-
recorded cases of BSE have occurred and an appropriate system of ing vaccination risk.
monitoring and reporting of BSE in animals has been imple-
mented. Alternatively, bovine material may be sourced from Competing interests
specific donor herds where animal health is routinely monitored.
None identified.
Use of manufacturing processes, such as heat sterilisation and
chemical treatment, reduces or removes BSE infectivity from
bovine products. References
1 World Health Organization. Vaccines, immunization and biologicals:
diseases and vaccines. The history of vaccination. Available at: http://
Live vaccines www.who.int/vaccines-diseases/history/history.shtml (accessed Dec
2005).
Non-living vaccines contain dead organisms or their components, 2 Australian Bureau of Statistics. Occasional paper: vaccination coverage in
which have been inactivated or killed by heat or chemical Australian children — ABS statistics and the Australian Childhood Immu-
treatment.35 In contrast, live attenuated vaccines contain live nisation Register (ACIR). Canberra: ABS, 2003. (ABS Catalogue No.
organisms that have lost the ability to cause serious disease, but 4813.0.55.001.)
retain sufficient antigenicity to cause an immune response that will 3 Gilbert GL, Gidding HF, Backhouse J, McIntyre PB. Varicella seropreva-
lence and vaccine uptake in preschool children [letter]. Med J Aust 2005;
protect against the original organism. These vaccines carry a risk of 182: 42.
organisms reverting to virulent forms that cause infection rather 4 Begg N, Ramsay M, White J, Bozoky Z. Media dents confidence in MMR
than immunity, but this is rare. Oral polio vaccine, for example, vaccine. BMJ 1998; 316: 561.
has been reported to produce about one case of vaccine-associated 5 Offit PA, Jew RK. Addressing parents’ concerns: do vaccines contain
paralytic poliomyelitis for every 2.4 million doses distributed.36 harmful preservatives, adjuvants, additives, or residuals? Pediatrics 2003;
112: 1394-1397.
Although not routinely used in Australia, the bacille Calmette– 6 Pichichero ME, Cernichiari E, Lopreiato J, Treanor J. Mercury concentra-
Guerin (BCG) vaccine may produce abscesses, regional lymphade- tions and metabolism in infants receiving vaccines containing thiomersal:
nopathy and disseminated disease in immunocompromised a descriptive study. Lancet 2002; 360: 1737-1741.
patients.37 Because of such risks, live vaccines are not recom- 7 National Health and Medical Research Council. The Australian Immunisa-
mended for routine use in pregnant or immunosuppressed tion Handbook. 8th ed. Canberra: NHMRC, 2003. Available at: http://
immunise.health.gov.au/handbook.htm (accessed Dec 2005).
patients.7
8 Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyper-
plasia, non-specific colitis, and pervasive developmental disorder in
children. Lancet 1998; 351: 637-641.
Formaldehyde
9 Murch SH, Anthony A, Casson DH, et al. Retraction of an interpretation.
Formaldehyde is used in the manufacture of killed vaccines to Lancet 2004; 363: 750.
inactivate the pathogenic effects of the organism, while preserving 10 Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing
vaccines and autistic spectrum disorder: a critical review of published
antigenicity.5 Concerns about the presence of formaldehyde are original data. Pediatrics 2004; 114: 793-804.
based on its toxicological profile and suggestions that it may act as 11 Patrizi A, Rizzoli L, Vincenzi C, et al. Sensitization to thimerosal in atopic
a carcinogen. Although industrial exposure to formaldehyde may children. Contact Dermatitis 1999; 40: 94-97.
be associated with certain cancers, cancer risk has not been fully 12 Vogt T, Landthaler M, Stolz W. Generalized eczema in an 18-month-old
established and may relate to degree of exposure.38 The current boy due to phenoxyethanol in DPT vaccine. Contact Dermatitis 1998; 38:
50-51.
standard for Australian vaccines is a maximum of 0.02% w/v of
13 Georgitis JW, Fasano MB. Allergenic components of vaccines and
free formaldehyde. During testing of Australian vaccines by the avoidance of vaccination-related adverse events. Curr Allergy Rep 2001;
TGA, the maximum formaldehyde concentration detected was 1: 11-17.
0.004% w/v.7 14 Finn TM, Egan W. Vaccine additives and manufacturing residuals in the
United States — licensed vaccines. In: Plotkin SA, Orenstein WA, editors.
Vaccines. 4th ed. Philadelphia: WB Saunders, 2004.
Latex 15 HogenEsch H. Mechanisms of stimulation of the immune response by
aluminum adjuvants. Vaccine 2002; 20 Suppl 3: S34-S39.
Patients may be exposed to latex during vaccination via its 16 Agency for Toxic Substances and Disease Registry. Toxicological profile
presence in syringe plungers and vial stoppers. Latex contains for aluminium. Atlanta: US Department of Health and Human Services,
numerous polypeptides, many of which may act as allergens; case Public Health Service, 1999. Available at: http://www.atsdr.cdc.gov/tox-
reports describe various immediate-type hypersensitivity reactions profiles/tp22.html (accessed Dec 2005).
occurring post-vaccination in patients with a history of latex 17 Kaaber K, Nielsen AO, Veien NK. Vaccination granulomas and aluminium
allergy: course and prognostic factors. Contact Dermatitis 1992; 26: 304-
allergy, including anaphylaxis.39 Removal of rubber stoppers or use 306.
of alternative delivery techniques are options for patients with a 18 Fawcett HA, Smith NP. Injection-site granuloma due to aluminum. Arch
history of serious latex allergy. Dermatol 1984; 120: 1318-1322.
174 MJA • Volume 184 Number 4 • 20 February 2006
CLINICAL UPDATE
19 Jefferson T, Rudin M, Di Pietrantonj C. Adverse events after immunisation www.medicines-partnership.org/our-publications/drugs-of-porcine-ori-
with aluminium-containing DTP vaccines: systematic review of the evi- gin (accessed Dec 2005).
dence. Lancet Infect Dis 2004; 4: 84-90. 31 Minor PD, Will RG, Salisbury D. Vaccines and variant CJD. Vaccine 2000;
20 Brenner A. Macrophagic myofasciitis: a summary of Dr. Gherardi’s pres- 19: 409-410.
entations. Vaccine 2002; 20 Suppl 3: S5-S6. 32 European Agency for the Evaluation of Medicinal Products. Questions
21 Rottem M, Shoenfeld Y. Vaccination and allergy. Curr Opin Otolaryngol and answers on bovine spongiform encephalopathies (BSE) and vac-
Head Neck Surg 2004; 12: 223-231. cines. London: European Medicines Agency, 2001. Available at: http://
22 James JM, Zeiger RS, Lester MR, et al. Safe administration of influenza www.emea.eu.int/pdfs/human/bwp/081901en.pdf (accessed Dec 2005).
vaccine to patients with egg allergy. J Pediatr 1998; 133: 624-628. 33 US Food and Drug Administration. Center for Biologics Evaluation and
23 Andrews RM, Kempe AE, Sinn KK, Herceg A. Vaccinating children with a Research. Questions and answers on bovine derived materials used in
history of serious reactions after vaccination or of egg allergy. Med J Aust vaccine manufacturing. Available at: http://www.fda.gov/cber/bse/
1998; 168: 491-494. bseqa.htm#a3 (accessed Mar 2005).
24 Castle P, Robertson JS. Summary and conclusion. In: Brown F, Cartwright 34 Therapeutic Goods Administration. Questions and answers on BSE risk
T, Horaud F, Spieser JM, editors. Animal sera, animal sera derivatives and associated with the use of materials of bovine origin during the manufac-
substitutes used in the manufacture of pharmaceuticals: viral safety and ture of vaccines. Available at: http://www.tga.gov.au/docs/html/bse-
regulatory aspects. Basel: Karger, 1999. faq.htm (accessed Mar 2005).
25 Grabenstein JD. Where medicine and religion intersect. Ann Pharmaco- 35 Egbert GB, Mascolo ED, Egan W. Vaccine manufacturing. In: Plotkin SA,
ther 2003; 37: 1338-1339. Orenstein WA, editors. Vaccines. Philadelphia: WB Saunders; 2004.
26 Rudd G. Is vaccination complicit with abortion? Ann Pharmacother 2003; 36 Burgess MA, McIntyre PB. Vaccine-associated paralytic poliomyelitis.
37: 1340-1341. Commun Dis Intell 1999; 23: 80-81.
27 Maher DP. Vaccines, abortion and moral coherence. Natl Cathol Bioeth Q 37 Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille
2002; 2: 51-67. Calmette-Guerin disease after vaccination: case report and review. Clin
28 Bashir A, Asif M, Lacey FM, et al. Concordance in Muslim patients in Infect Dis 1997; 24: 1139-1146.
primary care. Int J Pharmacy Pract 2001; 9(Suppl): R78. 38 Hauptmann M, Lubin JH, Stewart PA, et al. Mortality from solid cancers
29 Islamic Organization for Medical Sciences. The use of unlawful or among workers in formaldehyde industries. Am J Epidemiol 2004; 159:
juridically unclean substances in food and medicine. Question 104. Islam 1117-1130.
SET [website]. Available at: http://www.islamset.com/qa/index.html 39 Russell M, Pool V, Kelso JM, Tomazic-Jezic VJ. Vaccination of persons
(accessed Dec 2005). allergic to latex: a review of safety data in the Vaccine Adverse Event
30 Mynors G, Ghalamkari H, Beaumont S, et al. Informed choice in medicine Reporting System (VAERS). Vaccine 2004; 23: 664-667.
taking. Drugs derived from pigs and their clinical alternatives: an intro-
ductory guide. London: Medicines Partnership, 2004. Available at: http:// (Received 30 Jun 2005, accepted 5 Oct 2005) ❏
John Glenton Watson Obituary
BA, BEc, MB BS, FRACGP, FRACMA
A LOVED AND RESPECTED GENERAL PRACTITIONER in Sydney’s practice in South Coogee. He served many
Eastern Suburbs for many years, John Watson came from humble years as an Honorary Medical Officer in
beginnings. Excelling in many intellectual pursuits, he was an the Sydney Hospital general medicine and
eternal student. He loved teaching, and always had a close neurology outpatient clinics.
association with the University of Sydney and Sydney Hospital. John’s love of learning, teaching and
John was born in Sydney on 26 May 1917. He always wanted to patient care continued all his life. He was
be a doctor, but during the Depression no child of a poor family one of the first “radio talk-back doctors” to
could afford the fees. He won a Teachers’ College scholarship and answer listeners’ questions over the air. For
became a science teacher. While teaching by day, he attended many years he was a member of the Editorial Board of The Medical
evening classes in economics at the University of Sydney. Journal of Australia. He taught medicine and medical principles at
After serving in the Australian Military Forces from 1941 to the School of Occupational Therapy, and later became a director of
1946, John returned to teaching. At the same time, he completed the Sydney Medical Emergency Service Co-op, which provided an
Bachelor of Arts and Bachelor of Economics degrees as an evening out-of-hours locum service to GPs. He was also in great demand as
student, while saving to enrol in medicine. In 1948, he began to an after-dinner speaker and as a lecturer for the University of the
study medicine at the University of Sydney, supplementing his Third Age.
income by working as a bookmaker’s clerk. His sharpness as a His other interests, as a student and beyond, included debating
mathematician was valued, although his knowledge of and interest (the University of Sydney’s J G Watson trophy for debating was
in racing was nil! In 1950, he married Rose Wicks, also a teacher, named after him) and compering and scriptwriting for the annual
who helped to support him through medical school. Sydney University revue. He was President of the Sydney Univer-
After graduation in 1954, John was appointed Junior Resident at sity Union and later a student representative Fellow of the
Sydney Hospital. In 1956, approaching the age of 40, he estab- University Senate. He had a keen interest in many sports, includ-
lished a 24-hours-a-day, 7-days-a-week general practice in South ing surfing, fishing, cricket, rugby, athletics and hockey. At an
Coogee, living on the premises. Many of his patients became advanced age, he took up piano playing and landscape painting.
lifelong friends. John died on 6 July 2005 after several cerebral vascular
When Medicare was introduced, John felt he could not work incidents and eventual multiple organ failure. He was a truly good
under a system that “put the clock” on time spent with a patient, so man, who touched many people in his different roles. He will be
he went into hospital administration, becoming Deputy Superin- sadly missed by Rose, his sons John, Ian and Andrew, and their
tendent at Sydney Hospital. Although a capable administrator, he families and many friends.
found some aspects of the work very tedious and missed the
contact with patients, and so eventually returned to limited general Frederick O Stephens
MJA • Volume 184 Number 4 • 20 February 2006 175
You might also like
- Allergy, Immunity and Tolerance in Early Childhood: The First Steps of the Atopic MarchFrom EverandAllergy, Immunity and Tolerance in Early Childhood: The First Steps of the Atopic MarchHans Ulrich WahnRating: 3 out of 5 stars3/5 (1)
- Vaccine IngredientsDocument4 pagesVaccine IngredientsK. Banteilang NonglangNo ratings yet
- Swine Flu Vaccine FactsheetDocument4 pagesSwine Flu Vaccine Factsheetmuzaffaruz5932No ratings yet
- VACCINESDocument8 pagesVACCINESzilikajainNo ratings yet
- ConcernIngredientsAnimalIMAC200906V02Final PDFDocument2 pagesConcernIngredientsAnimalIMAC200906V02Final PDFFarhan Ullah KhanNo ratings yet
- CDC - Workshop On Thimerosal in Vaccines - 1999Document5 pagesCDC - Workshop On Thimerosal in Vaccines - 1999Eric UramNo ratings yet
- Journal of Dairy Science Volume Issue 2017 (Doi 10.3168 - jds.2017-12660) Kaczorek, E. Małaczewska, J. Wójcik, R. Rękawek, W. Siwic - Phenotypic and Genotypic Antimicrobial Susceptibility PatternDocument12 pagesJournal of Dairy Science Volume Issue 2017 (Doi 10.3168 - jds.2017-12660) Kaczorek, E. Małaczewska, J. Wójcik, R. Rękawek, W. Siwic - Phenotypic and Genotypic Antimicrobial Susceptibility Patternyudi poponNo ratings yet
- Schwalfenberg Et Al, 2018 (AJI 1)Document6 pagesSchwalfenberg Et Al, 2018 (AJI 1)Artika Muliany TindaonNo ratings yet
- Antibiotic GuidelinesDocument38 pagesAntibiotic GuidelinesKomang Adhi AmertajayaNo ratings yet
- Overdosed Babies:: Are Multiple Vaccines Safe?Document0 pagesOverdosed Babies:: Are Multiple Vaccines Safe?AprianaRohman100% (1)
- Evidence That Food Proteins in Vaccines Cause The Development of Food Allergies and Its Implications For Vaccine Policy 2329 6631 1000137Document3 pagesEvidence That Food Proteins in Vaccines Cause The Development of Food Allergies and Its Implications For Vaccine Policy 2329 6631 1000137Engkiong GoNo ratings yet
- Faq Vaccines ImmunisationDocument18 pagesFaq Vaccines ImmunisationAngela MitchelleNo ratings yet
- Project Brief Malawi WebDocument8 pagesProject Brief Malawi Websergio alvesNo ratings yet
- Parasite Control in Pasture-Grazed Dairy Cattle: Are We at The Edge of A Precipice?Document7 pagesParasite Control in Pasture-Grazed Dairy Cattle: Are We at The Edge of A Precipice?Oktrestu Dwi PutraNo ratings yet
- Defining Outcomes: HE Ournal of EdiatricsDocument2 pagesDefining Outcomes: HE Ournal of EdiatricsRizki Nur AmaliaNo ratings yet
- Public-Health Risks of Melamine in Milk Products: The Printed Journal Includes An Image Merely For IllustrationDocument2 pagesPublic-Health Risks of Melamine in Milk Products: The Printed Journal Includes An Image Merely For IllustrationLuiza Nicoleta MoglanNo ratings yet
- Peds 2010-0309 FullDocument11 pagesPeds 2010-0309 FullIkmah FauzanNo ratings yet
- Notes: Vaccines, Abortion, & Fetal TissueDocument5 pagesNotes: Vaccines, Abortion, & Fetal Tissuejdm81No ratings yet
- Final D Vaccines White Paper1Document7 pagesFinal D Vaccines White Paper1mariandNo ratings yet
- BTE722 - 2aDocument65 pagesBTE722 - 2aRakesh bhukyaNo ratings yet
- Proceedings of The 16th Italian Association of Equine Veterinarians CongressDocument10 pagesProceedings of The 16th Italian Association of Equine Veterinarians CongressCabinet VeterinarNo ratings yet
- AMR Profiles of Common Mastitis Pathogens On Canadian Dairy FarmsDocument14 pagesAMR Profiles of Common Mastitis Pathogens On Canadian Dairy FarmsApril LarasatiNo ratings yet
- Panchaud Et Al-2012-The Journal of Clinical Pharmacology-MinDocument9 pagesPanchaud Et Al-2012-The Journal of Clinical Pharmacology-MinAnna NutsubidzeNo ratings yet
- Pharmacy Daily For Tue 25 Mar 2014 - UK NIP Approves Bexsero, Mental Health Study, New Bleeding App, Letter To The Editor and Much MoreDocument3 pagesPharmacy Daily For Tue 25 Mar 2014 - UK NIP Approves Bexsero, Mental Health Study, New Bleeding App, Letter To The Editor and Much MorepharmacydailyNo ratings yet
- Ryeqo Epar Risk Management Plan Summary - enDocument5 pagesRyeqo Epar Risk Management Plan Summary - enUmaima faizNo ratings yet
- ThimerosalDocument73 pagesThimerosalBruno ReyesNo ratings yet
- Farmacología AntimastiticosDocument30 pagesFarmacología AntimastiticosAndres Alejandro CoralNo ratings yet
- Verizon Ebook Q3 3 Pediatric ToxicologyDocument9 pagesVerizon Ebook Q3 3 Pediatric ToxicologycesarNo ratings yet
- The Time of COVID - Phillip AltmanDocument107 pagesThe Time of COVID - Phillip AltmanYunSang ShinNo ratings yet
- Preventive Veterinary Medicine: E. Rollin, K.C. Dhuyvetter, M.W. OvertonDocument8 pagesPreventive Veterinary Medicine: E. Rollin, K.C. Dhuyvetter, M.W. OvertonBharathidhasan SelvarasuNo ratings yet
- Vaccine Friendly PlanDocument4 pagesVaccine Friendly PlanAna Sampaio89% (9)
- The Informative Value of An Overview On Antibiotic Consumption, Treatment Efficacy and Cost of Clinical Mastitis at Farm LevelDocument8 pagesThe Informative Value of An Overview On Antibiotic Consumption, Treatment Efficacy and Cost of Clinical Mastitis at Farm LeveljsolvarelaNo ratings yet
- 98 Aflatoxin Reduction in Milk and Milk Products PDFDocument35 pages98 Aflatoxin Reduction in Milk and Milk Products PDFTaj 825No ratings yet
- AddddDocument14 pagesAddddapi-547718836No ratings yet
- Monkeypox and Pregnancy - What Maternal-Fetal Medicine Subspecialists Need To KnowDocument7 pagesMonkeypox and Pregnancy - What Maternal-Fetal Medicine Subspecialists Need To KnowariniNo ratings yet
- Cost-Benefit Paratuberculosis: Analysis Vaccination Against Dairy CattleDocument5 pagesCost-Benefit Paratuberculosis: Analysis Vaccination Against Dairy CattlearavaNo ratings yet
- Austprescr 44 119Document5 pagesAustprescr 44 119Ingook SongNo ratings yet
- Vaccine Ingredients: What You Should KnowDocument2 pagesVaccine Ingredients: What You Should KnowKomsos - AG et al.No ratings yet
- Vaccination: Dairy Hub Training Booklets TitlesDocument8 pagesVaccination: Dairy Hub Training Booklets TitlesQais KaisraniNo ratings yet
- Vaccine Components Fact SheetDocument5 pagesVaccine Components Fact Sheetachuthasaran@11No ratings yet
- 57 - Adverse Drug Reactions in Breastfed Less Than ImaginedDocument16 pages57 - Adverse Drug Reactions in Breastfed Less Than ImaginedsasNo ratings yet
- Masitis Bovina Control Basado en Medicina PreventivaDocument4 pagesMasitis Bovina Control Basado en Medicina PreventivaDaniela BuenoNo ratings yet
- Postpartum Dysgalactia in SowsDocument4 pagesPostpartum Dysgalactia in SowsJuanita JordanNo ratings yet
- Edible Vaccines: Promises and Challenges: Vrinda M Kurup Jaya ThomasDocument12 pagesEdible Vaccines: Promises and Challenges: Vrinda M Kurup Jaya ThomasBelaNo ratings yet
- Current Treatment Aspects of Bovine Reproductive DisordersDocument10 pagesCurrent Treatment Aspects of Bovine Reproductive DisordersTatiana LageNo ratings yet
- Vesterlund Et Al, 2007 - Safety Assessment of Lactobacillus Strains PDFDocument7 pagesVesterlund Et Al, 2007 - Safety Assessment of Lactobacillus Strains PDFDanilo SilvaNo ratings yet
- Pharmatutor: Edible Vaccine - A Great Boon in Medicinal ScienceDocument4 pagesPharmatutor: Edible Vaccine - A Great Boon in Medicinal ScienceShailendra YadavNo ratings yet
- Vaccines and Population ControlDocument113 pagesVaccines and Population Controltheuselesseater100% (9)
- Nutfruit March 2016 IvarssonDocument3 pagesNutfruit March 2016 IvarssonFred EricNo ratings yet
- Annex 48 Clinical Guidelines For Covid in Malaysia 4th Edition 19102021 FinaleDocument175 pagesAnnex 48 Clinical Guidelines For Covid in Malaysia 4th Edition 19102021 FinaleEfendi AnuarNo ratings yet
- Cat HerniasDocument4 pagesCat HerniasbogdanotiNo ratings yet
- Vaccine Plan: The Dr. Paul ApprovedDocument9 pagesVaccine Plan: The Dr. Paul Approvedpaul popescu100% (1)
- Antibiotic Growth-Promoters in Food Animals: Peter HughesDocument25 pagesAntibiotic Growth-Promoters in Food Animals: Peter Hughesmekyno32No ratings yet
- A44 CMAJ Reducing The Pain of Childhood VaccinationDocument7 pagesA44 CMAJ Reducing The Pain of Childhood VaccinationGabriel CampolinaNo ratings yet
- Main Challenges in Poultry Farming: Hatchery Vaccination: Mohamed Faizal Abdul-CareemDocument25 pagesMain Challenges in Poultry Farming: Hatchery Vaccination: Mohamed Faizal Abdul-CareemN SipyonNo ratings yet
- ProceedingsDocument28 pagesProceedingsdjordjevetNo ratings yet
- Antiinfective Properties of Human MilkDocument6 pagesAntiinfective Properties of Human Milkkiko arrojasNo ratings yet
- Antiallergic Effects of ProbioticsDocument4 pagesAntiallergic Effects of ProbioticsputrinaraheswariNo ratings yet
- BIMA Oxford AZ Vaccine Detailed Position StatementDocument5 pagesBIMA Oxford AZ Vaccine Detailed Position StatementVegha NedyaNo ratings yet
- Probiotics and Preterm Infants A Position Paper.26 ShareDocument17 pagesProbiotics and Preterm Infants A Position Paper.26 Shareendy tovarNo ratings yet
- Histopathology Easy Mnemonic by ShahDocument18 pagesHistopathology Easy Mnemonic by ShahShah MohammedNo ratings yet
- Quick Quiz: Number of Yeast CellsDocument2 pagesQuick Quiz: Number of Yeast CellsVictor Barber SanchisNo ratings yet
- NCHSE Infection Control TESTDocument4 pagesNCHSE Infection Control TESTnurse1990No ratings yet
- Bio ProjectDocument25 pagesBio Projectlipi galotNo ratings yet
- ShingrixDocument6 pagesShingrixMarcus YoonNo ratings yet
- Portfolio IN Community Health Nursing: Submitted byDocument7 pagesPortfolio IN Community Health Nursing: Submitted byJay VillasotoNo ratings yet
- Maklumat Vaksinasi: Vaccination DetailsDocument2 pagesMaklumat Vaksinasi: Vaccination Detailsbd sinarbarakahNo ratings yet
- Indonesia National Immunization Program:: Dr. Prima Yosephine Epi Manager Moh IndonesiaDocument19 pagesIndonesia National Immunization Program:: Dr. Prima Yosephine Epi Manager Moh IndonesiaAstri KhairanaNo ratings yet
- 27 Best Coverage of Government - Suzy Davis - Katelyn Mary SkaggsDocument11 pages27 Best Coverage of Government - Suzy Davis - Katelyn Mary SkaggsSteve TaylorNo ratings yet
- The President Cory Aquino-HealthDocument4 pagesThe President Cory Aquino-HealthJesiah PascualNo ratings yet
- Thesis On Respiratory SystemDocument96 pagesThesis On Respiratory SystemDr Nimit ShahNo ratings yet
- Genomics BLAST AssignmentDocument12 pagesGenomics BLAST AssignmentDaniel Suarez100% (1)
- UICC Annual Report 2008Document48 pagesUICC Annual Report 2008schreamonn5515No ratings yet
- MRNA Vaccine Against Avian FluDocument5 pagesMRNA Vaccine Against Avian Fluanjum_niaziNo ratings yet
- Fernando - Sico - Elo - (181111010) Bahasa InggrisDocument2 pagesFernando - Sico - Elo - (181111010) Bahasa InggrisAsicoNo ratings yet
- Vaccines and Related Biological Products Advisory Committee MeetingDocument64 pagesVaccines and Related Biological Products Advisory Committee MeetingJim HoftNo ratings yet
- Brgy. Tonsuya Morbidity Week 36Document56 pagesBrgy. Tonsuya Morbidity Week 36Kathy OrtizNo ratings yet
- Social Exclusion Caste Health - A Review Based On The SocialDocument9 pagesSocial Exclusion Caste Health - A Review Based On The SocialSanthosh TekumalNo ratings yet
- Part III Final CDX 2bDocument15 pagesPart III Final CDX 2bBethrice MelegritoNo ratings yet
- TrepDocument50 pagesTrepMiguel Adrian GaonaNo ratings yet
- Sentimental Medicine PDFDocument8 pagesSentimental Medicine PDF杨飞燕No ratings yet
- RX Guide Delivering GoodsDocument9 pagesRX Guide Delivering GoodsjesakuNo ratings yet
- Other: RegionDocument4 pagesOther: RegionJoseph RepotenteNo ratings yet
- Im SQ Admin PDFDocument2 pagesIm SQ Admin PDFAlvin JjNo ratings yet
- Prioritization of Mabs During Resource Shortages 20211229Document3 pagesPrioritization of Mabs During Resource Shortages 20211229News10NBCNo ratings yet
- Aerobic Non-Spore Forming Gram-Positive BacilliDocument31 pagesAerobic Non-Spore Forming Gram-Positive BacilliCagar Irwin TaufanNo ratings yet
- Late Shri Vinod Kumar MittalDocument6 pagesLate Shri Vinod Kumar MittaldasohamathNo ratings yet
- My Fiel Report 2018Document24 pagesMy Fiel Report 2018Popyeninawa HanyehaitilaNo ratings yet
- 2 Circle Venn Diagram 1Document1 page2 Circle Venn Diagram 1Mica RothenseeNo ratings yet
- Bacterial Sinusitis in Children: AcuteDocument11 pagesBacterial Sinusitis in Children: AcutefriscahalimNo ratings yet
- Do You Believe in Magic?: The Sense and Nonsense of Alternative MedicineFrom EverandDo You Believe in Magic?: The Sense and Nonsense of Alternative MedicineNo ratings yet
- The Wisdom of Plagues: Lessons from 25 Years of Covering PandemicsFrom EverandThe Wisdom of Plagues: Lessons from 25 Years of Covering PandemicsRating: 4.5 out of 5 stars4.5/5 (6)
- Uncontrolled Spread: Why COVID-19 Crushed Us and How We Can Defeat the Next PandemicFrom EverandUncontrolled Spread: Why COVID-19 Crushed Us and How We Can Defeat the Next PandemicNo ratings yet
- Summary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisFrom EverandSummary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (9)
- Fatal Conveniences: The Toxic Products and Harmful Habits That Are Making You Sick—and the Simple Changes That Will Save Your HealthFrom EverandFatal Conveniences: The Toxic Products and Harmful Habits That Are Making You Sick—and the Simple Changes That Will Save Your HealthRating: 4 out of 5 stars4/5 (7)
- The Gut-Immune Connection: How Understanding the Connection Between Food and Immunity Can Help Us Regain Our HealthFrom EverandThe Gut-Immune Connection: How Understanding the Connection Between Food and Immunity Can Help Us Regain Our HealthNo ratings yet
- Deaths of Despair and the Future of CapitalismFrom EverandDeaths of Despair and the Future of CapitalismRating: 4.5 out of 5 stars4.5/5 (30)
- Summary: The Real Anthony Fauci: Bill Gates, Big Pharma, and the Global War on Democracy and Public Health by Robert F. Kennedy Jr: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Real Anthony Fauci: Bill Gates, Big Pharma, and the Global War on Democracy and Public Health by Robert F. Kennedy Jr: Key Takeaways, Summary & Analysis IncludedNo ratings yet
- The Truth about Wuhan: How I Uncovered the Biggest Lie in HistoryFrom EverandThe Truth about Wuhan: How I Uncovered the Biggest Lie in HistoryRating: 4 out of 5 stars4/5 (6)
- Sickening: How Big Pharma Broke American Health Care and How We Can Repair ItFrom EverandSickening: How Big Pharma Broke American Health Care and How We Can Repair ItRating: 4 out of 5 stars4/5 (9)
- The Hair Color Mix Book: More Than 150 Recipes for Salon-Perfect Color at HomeFrom EverandThe Hair Color Mix Book: More Than 150 Recipes for Salon-Perfect Color at HomeRating: 3.5 out of 5 stars3.5/5 (7)
- The Inescapable Immune Escape PandemicFrom EverandThe Inescapable Immune Escape PandemicRating: 5 out of 5 stars5/5 (1)
- War on Ivermectin: The Medicine that Saved Millions and Could Have Ended the PandemicFrom EverandWar on Ivermectin: The Medicine that Saved Millions and Could Have Ended the PandemicRating: 4 out of 5 stars4/5 (7)
- Microbiological Quality of FoodsFrom EverandMicrobiological Quality of FoodsL SlanetzNo ratings yet
- Profiles of the Vaccine-Injured: "A Lifetime Price to Pay"From EverandProfiles of the Vaccine-Injured: "A Lifetime Price to Pay"Rating: 3.5 out of 5 stars3.5/5 (3)
- There Are No Accidents: The Deadly Rise of Injury and Disaster—Who Profits and Who Pays the PriceFrom EverandThere Are No Accidents: The Deadly Rise of Injury and Disaster—Who Profits and Who Pays the PriceRating: 4.5 out of 5 stars4.5/5 (15)
- Vaccines Did Not Cause Rachel's Autism: My Journey as a Vaccine Scientist, Pediatrician, and Autism DadFrom EverandVaccines Did Not Cause Rachel's Autism: My Journey as a Vaccine Scientist, Pediatrician, and Autism DadRating: 4.5 out of 5 stars4.5/5 (3)
- Epidemics and Society: From the Black Death to the PresentFrom EverandEpidemics and Society: From the Black Death to the PresentRating: 4.5 out of 5 stars4.5/5 (9)
- Arthritis Diet: Anti-inflammatory Diet for Arthritis Pain ReliefFrom EverandArthritis Diet: Anti-inflammatory Diet for Arthritis Pain ReliefNo ratings yet
- Make America Healthy Again: How Bad Behavior and Big Government Caused a Trillion-Dollar CrisisFrom EverandMake America Healthy Again: How Bad Behavior and Big Government Caused a Trillion-Dollar CrisisNo ratings yet
- The Atlas of Disease: Mapping Deadly Epidemics and Contagion from the Plague to the CoronavirusFrom EverandThe Atlas of Disease: Mapping Deadly Epidemics and Contagion from the Plague to the CoronavirusRating: 4.5 out of 5 stars4.5/5 (10)
- The Gut-loving Cookbook: Over 65 deliciously simple, gut-friendly recipes from The Gut StuffFrom EverandThe Gut-loving Cookbook: Over 65 deliciously simple, gut-friendly recipes from The Gut StuffNo ratings yet
- Panic Attack: Playing Politics with Science in the Fight Against COVID-19From EverandPanic Attack: Playing Politics with Science in the Fight Against COVID-19Rating: 4.5 out of 5 stars4.5/5 (10)