Professional Documents
Culture Documents
Trypanosome Species, Morphology, Disease Caused
Uploaded by
Arielle VidalOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Trypanosome Species, Morphology, Disease Caused
Uploaded by
Arielle VidalCopyright:
Available Formats
Species
Morphology
Disease caused
All trypanosomes are heteroxenous or at least are transmitted by vectors. Various species pass through ama blood and tissue fluids, but some occupy intracellular habitats. The majority are transmitted by blood-feeding i hosts. Salivaria: develops in the anterior portions of the digestive tract (anteri
Trypanosoma brucei rhodesiense
Subspecies of Trypanosoma brucei ; morphologically indistinguishable, vary in infectivity for diff. species of hosts, produce somewhat different pathological syndromes, etiological agents of African sleeping sickness; there are physiological differences between subspecies; differ in pathogenesis, growth rate, and biology
causes a more acute type of infection
Trypanosoma brucei gambiense
Causes a chronic form of sleeping sickness (invasion of CNS, multiply, and enter intercellular spaces in brain) ; native game animals serve as reservoirs for Rhodesian trypanosomiasis not Gambian
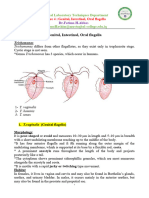
Trypomastigotes are found in circulating blood. They are slender, and posterior end is pointed. Free flagellum is moderately long, undulating membrane is narrow with only 2-3 undulation at a tie along its length. Trypanosoma cruzi Chagas' disease - manifests acute and chronic phases
Trypanosoma cruzi
Chagas' disease - manifests acute and chronic phases
Kinetoplast is subterminal and is the largest of any trypanosome, sometimes causing the body to bulge out. Commonly dies in a question marke shape (retained in stained smears). Amastigotes develop in muscles and other tissues. They are spheroid and occur in clusters.
Leishmanias are heteroxenous. Part of life cycle is spent in a sand fly gut where they become promastigotes. R found. Cause a complex of diseases called leishmaniasis, which is a zoonosis with a wild animal reservoir. Live clinical manifestations, drug treatment may precipitate a subsequent clinical manifestation quite different from th parasites pass through midgut or hindgut, where they transform into procyclic promastigotes that attach to the g lining and forming plaques (hemidesmosomes) > 8th day: flagellates metamorphose into slender, active, metac differing only in size. Only the nucleus and very large kinetoplast can be seen, and cytoplasm appears vacuolat properties, especially in their membrane components, and in the mechanism by which they survive.
Leishmania donovani
Symptoms
vectors. Various species pass through amastigote, promastigote stages, with other forms developing in the invertebrate host. Parasites of all vertebrate c majority are transmitted by blood-feeding invertebrates. Divided into two broad groups: Salivaria and Stercoraria, based on characteristics of their devel nterior portions of the digestive tract (anterior station development); Stercocaria: develops in vector's hindgut (posterior station development).
Small sore (chancre) often develops at the site where metacyclic trypanosomes are inoculated. This disappears after 1-2 weeks > protozoa gain entry to the blood and lymph channels > reproduce rapidly, producing parasitemia and invading all organs of the body. Intermittent periods of fever at early stages > increase in number of trypanosomes in circulating blood, increase in swelling of lymph nodes, generalized pain, headache, weakness, and cramps
Rarely invade the nervous system.
Acute phase - most common and sever among children. Inoculation of trypanosomes from the bug feces into the wound > Local inflammation > small red nodule (chagoma) with swelling of regional lymph nodes > trypanosomes enter through the conjunctiva of the eye > edema of the eyelid and conjunctiva and swelling of the preauricular lymph node (Romana's sign) > pseudocysts may be found in almost any organ of the body > anemia, loss of strength, nervous disorders, chills, muscle and bone pain, varying degress of heart failure. Heart muscle is usually invaded, with up to 80% ganglion cells lost.
Chronic stage - most often seen in adults. Spectrum of symptoms is primarily the result of CNS and PNS dysfunction. Part of the inefficency in heart function is caused by loss of muscle tone resulting from destroyed nerve ganglia.Heart becomes enlarged and flabby.
y gut where they become promastigotes. Remainder of life cycle is completed in vertebrate (mammal) tissues where only amastigotes (Leishman-Donov a zoonosis with a wild animal reservoir. Live in inside macrophages of vertebrate host, amastigotes in marcophages reside in phagolysosomes, species di clinical manifestation quite different from that of the original infection (post-kala-azar dermal leishmanoid). Sand flies ingest amastigotes from blood of procyclic promastigotes that attach to the gut and replicate by binary fission > 4th or 5th day after feeding: promastigotes move to the esophagus and pha s metamorphose into slender, active, metacyclic promastigotes that are injected into the next blood meal. All amastigotes look similar in vertebrate tissues n be seen, and cytoplasm appears vacuolated in stained preparations. Short axoneme is visible within the cytoplasm. Amastigotes of species differ in their chanism by which they survive.
Pathogenesis
Distribution
ages, with other forms developing in the invertebrate host. Parasites of all vertebrate classes. Most live in o two broad groups: Salivaria and Stercoraria, based on characteristics of their development in the insect Stercocaria: develops in vector's hindgut (posterior station development).
Invade the CNS > chronic, sleeping sickness stage of infection > increase in apathy and mental dullness with accompanied disturbances of coordination > tremor of the tongue, hands, and trunk > paralysis and convulsions > sleepiness> coma > death. Death may be due to malnutrition, pneumonia. Heart failure, and other parasitic infections. Central and East Central Africa Can enter microvascular endothelial cells of the blood-brain barrier. In acute infections of small animals: death occurs rapidly with a high level of parasitemia, mortality results from overall disruption of physiological processes. In humans: neurological involvement results in demyelinating encephalitis, accompanied by dementia, occasional hallucinations, and decreased consciousness.
Causes a more rapid course toward death (lymph nodes in the neck, groin, and legs swell up West Central and [Winterbottom's sign]). Infection causes rapid weight loss and heart involvement, causes no Central Africa somnambulism or other protracted nervous disorders found with T. b. gambiense
Entrance of metacyclic trypanosomes into cells of subcuteanous tissue > acute local inflammatory reaction > 1-2 weeks after: parasites spread to regional lymph nodes and begin to multiply in cells that phagocytose them. Intracellular amastigotes undergo repeated divisions to form large numbers of parasites, producing a pseudocyst > generalized parasitemia > parasite invades almost every type of tissue in the body (show a particular preference for muscle and nerve cells). South and Central America
South and Central America
Reversion to amastigote, pseudocyst formation, retransformation to trypomastigote, and pseudocyst rupture are repeated in newly invaded cells. Rupture of pseudocyst > acute inflammatory response with degeneration and necrosis of nerve cells (especially of ganglion cells). Degeneration is the indirect result of parasitism of supporting cells, such as glial cells and macrophages.
completed in vertebrate (mammal) tissues where only amastigotes (Leishman-Donovan [L-D] bodies) are of vertebrate host, amastigotes in marcophages reside in phagolysosomes, species differ in terms of on (post-kala-azar dermal leishmanoid). Sand flies ingest amastigotes from blood of infected animal > ry fission > 4th or 5th day after feeding: promastigotes move to the esophagus and pharynx, attaching to the are injected into the next blood meal. All amastigotes look similar in vertebrate tissues: spheroid to ovoid, ns. Short axoneme is visible within the cytoplasm. Amastigotes of species differ in their biochemical
Insect Vectors
Form structure
Tend to be pleiomorphic in natural infections (in vertebrate host): from long, relatively slender trypomastigotes with a long free flagellum through intermediate forms to short, stumpy individuals with no free flagellum; small kinetoplast usually near the posterior end; undulating membrane is Glossina morsitans, G. pallidipes, G. conspicuous swynnertomi Long, slender trypomastigotes have a single, simple mitochondrion extending anteriorly from their kinetoplast; cristae are few, short, and tubular
G. palpalis and G. tachinoides
conenosed bugs; reduviid bugs known to defecate on host's skin, feces contain metacyclic tryapansomes; triatomine bugs
Undulating membrane and flagellum disappear soon after parasite enters host. Intermediate forms (promastigotes and epimastigotes) can be seen in the interstitial spaces. Some complete metamorphosis into trypomastigotes and find their way into the blood.
Panstrongylus megistus, Triatoma sordida, T. brasiliensis (Brazil); T. infestans (Chile and Uruguay); Rhodnius prolixus (northern South America); T. dimidiata (Central America); T. barberi (Mexico); Dipetalogaster maxius (southern Baja California)
soon after parasite enters host. Intermediate forms (promastigotes and epimastigotes) can be seen in the interstitial spaces. Some complete metamorphosis into trypomastigotes and find their way into the blood.
Intermediate hosts and vectors are sand flies. Old World: Phlebotomus and Sergentomyia ; New World: Lutzomyia , Brumptomyia , and Warileya
Form function
Locomotor organelles
Reproduction
Flagellum -
Kinetoplast movement away from the posterior end in the midgut trypomastigote and anterior to the nucleus in the epimastigote reflects the elaboration of the posterior section of mitochondrion
Flagellum
Asexual reproduction in fly vector
Mitochondrion "pushes" kinetoplast forward
Flagellum
Trypomastigotes, although abundant in the blood in early infections, do not reproduce until they have entered a cell and have transformed into amatisgotes. Most frequently invaded are cells of the spleen, liver, and lymphatics and cells in cardiac, smooth, and skeletal muslces. Nervous system, skin, gonads, intestinal mucosa, bone marrow, and placenta also are infected.
Flagellum
Trypanosomes can actively penetrate host cells, but they may also enter through phagocytosis by host macrophages. Repeated binary fission produces many amastigotes, killing the cell > lysis > protozoa attack other cells.
Life Cycle
Encystment
Blood meal > locates in posterior section of midgut: multiplies in trypomastigote form (10 days) > migrate to foregut (12th - 20th day) > migrate to esophagus > pharynx > hypopharynx > salivary glands: become epimastigotes, attach to host cells or lie free in lumen > [asexual generations] > become metacyclic trypomastigotes (small, stumpy and lack a free flagellum; infective to vertebrates) {FLY: complete in 15 - 35 days} > in vertebrates: multiply as trypomastigotes in blood and lymph
Do not invade or live within cells but inhabit the connective tissue spaces within various organs and the reticular tissue spaces of the spleen and lymph nodes. Abundant in lymph vessels and Short, stumpy forms are the only ones infective to tsetse flies, and the intermediate intercellular spaces of the brain. forms are transitional from the long, slender noninfective forms. Transition is marked by increasing elaboration of the mitochondrion.
In vertebrate hosts: live in the blood, lymph, spleen, and cerebrospinal fluid.
Triatomine bugs ingest trypomastigotes > trypomastigotes pass through posterior portion of insect's midgut > become short epimastigotes > longitudinal fission > long, slender epimastigotes. 8-10 days after infection: short metacyclic trypomastigotes found in insect rectum.
Pseudocysts - cyst-like pockets of parasites that form in muscle cells.
Triatomine bugs ingest trypomastigotes > trypomastigotes pass through posterior portion of insect's midgut > become short epimastigotes > longitudinal fission > long, slender epimastigotes. 8-10 days after infection: short metacyclic trypomastigotes found in insect rectum.
Pseudocysts - cyst-like pockets of parasites that form in muscle cells.
Recent Studies
Feeding and metabolism
Excretion and osmoregulation
Strain 1: passed by syringe from one vertebrate host to another; becomes monorphic; slender trypomastigotes no longer infective to tsetse fles; cannot be cultivated in vitro , morphology and metabolism correspond to those in natural infections; very active consume substantial amounts of glucose and oxygen
Depend entirely on glycolysis: degrade glucose > pyruvate only; has no tricarboxylic acid cycle or oxidative phosphorylation by way of the classical cytochrome system (requires oxygen, not sensitive to cyanide)
Strain 2: placed in vitro culture systems; morphology and metabolism revert to those found in fly midgut; kinetoplast farther from posterior end and close to nucleus
Blood clot in vector's midgut: complete degradation of glucose through glycolysis, the tricarboxylic acid cycle, and the cyanide-sensitive cytochrome system
Oxygen consumption of blood and intracellular forms is the same as that of culture forms. Bloodstream forms have Krebs cycle and classical cytochrome system.
T. cruzi is a "partial aerobic fermenter". Some of the glucose carbon consumed is degraded completely to carbon dioxide.
Oxygen consumption of blood and intracellular forms is the same as that of culture forms. Bloodstream forms have Krebs cycle and classical cytochrome system.
It excretes a substantial portion as succinate and acetate.
Endosymbionts
Species
Morphology
Disease caused
Symptoms
Pathogenesis
Distribution
Insect Vectors
Form structure
Form function
Locomotor organelles
Reproduction
Life Cycle
Encystment
Recent Studies
Feeding and metabolism
Excretion and osmoregulation
Endosymbionts
You might also like
- Blood and Tissue Flagellates: Epidemiology and CharacteristicsDocument97 pagesBlood and Tissue Flagellates: Epidemiology and Characteristicsfeyisa girma50% (2)
- Clinical Parasitology OutlineDocument5 pagesClinical Parasitology OutlineLynneth Mae Beranda CorpusNo ratings yet
- Wild Foods For Wise Women by Susun WeedDocument6 pagesWild Foods For Wise Women by Susun Weedclaricecaps100% (1)
- Medical Parasitology: Intestinal and Blood ParasitesDocument24 pagesMedical Parasitology: Intestinal and Blood ParasitesSrijan BhattaraiNo ratings yet
- Parasitology: MetazoansDocument28 pagesParasitology: MetazoansDanessa AlavataNo ratings yet
- Autophagy and CancerDocument349 pagesAutophagy and CancerSohibun21No ratings yet
- Surgical InfectionsDocument310 pagesSurgical InfectionsOmar Ed ChavezNo ratings yet
- Parasitology NotesDocument59 pagesParasitology NotesGiorgos Doukas KaranasiosNo ratings yet
- Medical Protozoology. 1Document55 pagesMedical Protozoology. 1miniwhiteyNo ratings yet
- Submitted By: Group 6 MT 3BDocument49 pagesSubmitted By: Group 6 MT 3BChristine Joy TanglaoNo ratings yet
- Blood and Tissue ProtozoansDocument12 pagesBlood and Tissue ProtozoansHumayun ArshadNo ratings yet
- Blood Tissue and FlagellatesDocument15 pagesBlood Tissue and FlagellatesHughNo ratings yet
- Parasitology ProtozoaDocument132 pagesParasitology ProtozoaMikay Cutaran0% (1)
- Introduction To: ParasitesDocument32 pagesIntroduction To: ParasitesJaznMonNo ratings yet
- Cardiac Case Study NDDocument11 pagesCardiac Case Study NDapi-313165458No ratings yet
- Atlas Electrónico de ParasitologíaDocument650 pagesAtlas Electrónico de ParasitologíaSoledad Rod100% (1)
- Hemofl - AgellatesDocument46 pagesHemofl - Agellatesمحمد رحيم حسن محمودNo ratings yet
- Parasitology NotesDocument38 pagesParasitology NotesEdoardo CitarellaNo ratings yet
- Blood and Tissue FlagellatesDocument5 pagesBlood and Tissue FlagellatesChristine BuenNo ratings yet
- Medical Parasitology: Blood & Tissue ProtozoaDocument17 pagesMedical Parasitology: Blood & Tissue ProtozoaIbraheem YesharNo ratings yet
- Hemoflagellates Lecture ST 2023 2024Document29 pagesHemoflagellates Lecture ST 2023 2024ibraheem.barzengiNo ratings yet
- ParásitosDocument26 pagesParásitosALAN FERNANDO GONZALEZ SERRANONo ratings yet
- Blood Born ProtozoansDocument194 pagesBlood Born ProtozoansMitzie NiñaNo ratings yet
- Biology Parasitology Notes on Parasitic Protozoa & Their Life CyclesDocument29 pagesBiology Parasitology Notes on Parasitic Protozoa & Their Life CyclesUsman Ali KhanNo ratings yet
- Visceral Leishmaniasis Parasite Life Cycle and Disease StagesDocument4 pagesVisceral Leishmaniasis Parasite Life Cycle and Disease StagesDennis ValdezNo ratings yet
- 8.0 HaemoflagellatesDocument11 pages8.0 HaemoflagellatesHenry KarokiNo ratings yet
- Blood & Tissue Protozoa The HemoflagellatesDocument19 pagesBlood & Tissue Protozoa The Hemoflagellatessetya setianaNo ratings yet
- Hemo Flagellate SDocument23 pagesHemo Flagellate SJames Carbonell Dela PeñaNo ratings yet
- CU-Parasitology 3 (Protozoa II)Document16 pagesCU-Parasitology 3 (Protozoa II)SallyCroyNo ratings yet
- 2 & 3 Protozoa and MalariaDocument22 pages2 & 3 Protozoa and Malariaمصطفي خندقاويNo ratings yet
- TrypanosomiasisDocument50 pagesTrypanosomiasisUlan RakhmatovNo ratings yet
- Parasite Trypanosoma GambienseDocument5 pagesParasite Trypanosoma GambienseSabahat LatifNo ratings yet
- Trypanosoma GambienesDocument5 pagesTrypanosoma Gambienesapi-481318101No ratings yet
- Life Cycle of Trypanosoma cruziDocument9 pagesLife Cycle of Trypanosoma cruziAL BURJ AL THAKINo ratings yet
- Trypanosomiasis in CamelsDocument20 pagesTrypanosomiasis in CamelsAnugat Jena0% (1)
- Vpa 222Document12 pagesVpa 222Sanjay KumarNo ratings yet
- Morfologi Leishmania DonovaniDocument2 pagesMorfologi Leishmania DonovaniafitaNo ratings yet
- Activity 2 Blood and Tissue ProtozoansDocument42 pagesActivity 2 Blood and Tissue ProtozoansRaven TolentinoNo ratings yet
- Leucocytozoon and Hepatozoon Parasites in Birds and DogsDocument37 pagesLeucocytozoon and Hepatozoon Parasites in Birds and DogsLe KhaiNo ratings yet
- PDF DocumentDocument6 pagesPDF DocumentPrecious Maridel CabugaNo ratings yet
- Helminthes: / Medical Laboratory TechnologyDocument17 pagesHelminthes: / Medical Laboratory Technologyصفا رياض محمد /مسائيNo ratings yet
- Flatworms 1Document31 pagesFlatworms 1GuteNo ratings yet
- A. Classification of ParasitesDocument8 pagesA. Classification of ParasitesRioNo ratings yet
- Haemoflagellates 2Document41 pagesHaemoflagellates 2RaunaNo ratings yet
- Tissue Mastigophora Medics 2Document83 pagesTissue Mastigophora Medics 2MarvellousNo ratings yet
- Parasitology Review 1Document20 pagesParasitology Review 1sdillon28No ratings yet
- UntitledDocument51 pagesUntitledHesham MogicoNo ratings yet
- PROTOZOANS_PARASITESDocument29 pagesPROTOZOANS_PARASITESpotatopurple009No ratings yet
- Protozoal Infections 29 April 2013Document87 pagesProtozoal Infections 29 April 2013Nive KojNo ratings yet
- Pathogenic Flagellates: (Quick Review) and Still Capture The Pertinent InformationDocument23 pagesPathogenic Flagellates: (Quick Review) and Still Capture The Pertinent Informationryanjt178No ratings yet
- ProtozoaDocument25 pagesProtozoanupur.kmcNo ratings yet
- E Learning SensoriDocument15 pagesE Learning SensoriadnajaniNo ratings yet
- Genital, Intestinal, Oral FlagellaDocument7 pagesGenital, Intestinal, Oral Flagellaxofoh43003No ratings yet
- Parasitology Lec5Document13 pagesParasitology Lec5ao868598No ratings yet
- Aust Clinical Parasitology CLS 450: Dr. Renée Zakhia Rzakhia@aust - Edu.lbDocument33 pagesAust Clinical Parasitology CLS 450: Dr. Renée Zakhia Rzakhia@aust - Edu.lbChristine KamaleddineNo ratings yet
- Unit 9.N. Blood and Tissue FlagellatesDocument41 pagesUnit 9.N. Blood and Tissue FlagellatesMichael DawitNo ratings yet
- Life Cycle, Pathogenicity, Causes, Symptoms and Control of LeishmaniaDocument24 pagesLife Cycle, Pathogenicity, Causes, Symptoms and Control of LeishmaniaAditi TanwarNo ratings yet
- Parasites PetalsDocument115 pagesParasites PetalsMcarl MatelNo ratings yet
- Theme 18Document13 pagesTheme 18Sunaina MahaviraNo ratings yet
- Parasitic FlagellatesDocument21 pagesParasitic Flagellatesblue_blooded23100% (1)
- ParasitologyDocument53 pagesParasitologyshakila786No ratings yet
- KINGDOM PROTISTA - BSEdIIIPDocument40 pagesKINGDOM PROTISTA - BSEdIIIPKarl Francis GarciaNo ratings yet
- Camp's Zoology by the Numbers: A comprehensive study guide in outline form for advanced biology courses, including AP, IB, DE, and college courses.From EverandCamp's Zoology by the Numbers: A comprehensive study guide in outline form for advanced biology courses, including AP, IB, DE, and college courses.No ratings yet
- Arterial DiseaseDocument191 pagesArterial DiseaseAura DiscyacittaNo ratings yet
- Study DesignDocument130 pagesStudy Designephremtigabie7No ratings yet
- Figure 1. New Criteria For AKI Diagnosis Are Displayed. in Order To Diagnose AKIDocument8 pagesFigure 1. New Criteria For AKI Diagnosis Are Displayed. in Order To Diagnose AKIMayra Alejandra Prada SerranoNo ratings yet
- Cellular Aberrations Guide to Cancer DiagnosisDocument5 pagesCellular Aberrations Guide to Cancer DiagnosisIrish Eunice Felix100% (1)
- Multiple Births Carrying Multiples:-Triplets, Quadruplets, Quintuplets, SextupletsDocument5 pagesMultiple Births Carrying Multiples:-Triplets, Quadruplets, Quintuplets, SextupletsSheryll Almira HilarioNo ratings yet
- Corpectomy Cage Surgical TechniquesDocument18 pagesCorpectomy Cage Surgical TechniquesJulian VargasNo ratings yet
- EMT Training at Mansion Mandiri HotelDocument4 pagesEMT Training at Mansion Mandiri Hotelyuna triazNo ratings yet
- Understanding Hepatic Encephalopathy (HE) Symptoms, Causes and TreatmentDocument3 pagesUnderstanding Hepatic Encephalopathy (HE) Symptoms, Causes and TreatmentSuhas KandeNo ratings yet
- Chapter 4 Communicable DiseaseDocument33 pagesChapter 4 Communicable DiseaseAyro Business CenterNo ratings yet
- Literature Review On Premature BabiesDocument4 pagesLiterature Review On Premature Babiesc5eakf6z100% (1)
- Introduction To Epidemiology of Infection Diseases PDFDocument17 pagesIntroduction To Epidemiology of Infection Diseases PDFRovaidKhanNo ratings yet
- CLINICAL CHEMISTRY 2 Tumor MarkersDocument3 pagesCLINICAL CHEMISTRY 2 Tumor MarkersSuzzaine EniazzusNo ratings yet
- Rohini 59284010117Document21 pagesRohini 59284010117narasimmanbiomedicalNo ratings yet
- الهيئة 3Document10 pagesالهيئة 3mohsenNo ratings yet
- 2013 Pankaj R BodadeDocument8 pages2013 Pankaj R BodadeGeorge StoicaNo ratings yet
- Presented by DR Rahul D AgrawalDocument64 pagesPresented by DR Rahul D AgrawalRahul AgrawalNo ratings yet
- Definition and Characteristics of AntibioticsDocument4 pagesDefinition and Characteristics of AntibioticsfirozbangladeshNo ratings yet
- Inguinal LAPDocument12 pagesInguinal LAPDarawan MirzaNo ratings yet
- Muhammad YounasDocument4 pagesMuhammad YounasHamid IqbalNo ratings yet
- MSN I 20.4.2020 FN TyphoidDocument20 pagesMSN I 20.4.2020 FN TyphoidDr. DhaneshNo ratings yet
- Baby SensesDocument2 pagesBaby SensesCamille LiqueNo ratings yet
- How Food Handlers Can Spread IllnessDocument2 pagesHow Food Handlers Can Spread IllnessSavitri Maharani BudimanNo ratings yet
- My SukshamvyamDocument6 pagesMy SukshamvyamsurendermadaanNo ratings yet
- Data FinalDocument72 pagesData FinalAchsan KamalNo ratings yet
- How to treat and prevent breast engorgementDocument1 pageHow to treat and prevent breast engorgementkurniaNo ratings yet
- Randomized, Placebo-Controlled Trial of Xyloglucan and Gelose For The Treatment of Acute Diarrhea in ChildrenDocument8 pagesRandomized, Placebo-Controlled Trial of Xyloglucan and Gelose For The Treatment of Acute Diarrhea in ChildrenvalenciaNo ratings yet