Professional Documents
Culture Documents
CHM 580 Exp Aas
Uploaded by
Azreen AnisOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHM 580 Exp Aas
Uploaded by
Azreen AnisCopyright:
Available Formats
Objective : To determine Cadmium (Cd) and Chromiun (Cr) in plant tissue using absorption spectroscopy Introductions : Cadmium is well
known to be one of the most toxic heavy elements for animals. It has recently become a serious problem that rice grains contain cadmium in some area of Japan. On the other hand, some types of plants can grow in contaminated soils and absorb a large amount of cadmium in their bodies. Such hyperaccumulator plants are expected to be used for remediation of environments. However, the accumulation mechanism has not yet been revealed, with the elemental distribution of cadmium and transportation during uptake remaining unclear. Cadmium appear to play a very pivotal role in thyroid disease, it is a very unique mineral. It is extremely toxic and has toxic biological effects at concentrations smaller than almost any commonly found mineral. An environmental poison found in water, on our food and in the air. It's found in processed grains, dairy products, meats, fish, fertilizers, auto exhaust, cigarette smoke, batteries, solder and dentures. It disrupts the absorption of other minerals and tends to settle in the heart and right kidney and affects proper functioning of several enzymes. Whereas for Chromium, taken in the right quantity, chromium has immense health benefits. It is available in extremely low quantities in animal and plant tissues which is why it is called a trace metal. Some of the sources of chromium are brewers yeast, coffee, tea, cereals, potatoes, peas, oysters, rye, thyme, processed meats, whole grains, and beer. Chromium helps metabolize carbohydrates. It monitors blood sugar levels, and helps stabilize blood sugar. It can also prevent hypertension or high blood pressure. Although trials are still being conducted, chromium compounds are considered helpful in preventing memory loss and in treating Alzheimers disease. Procedure : Day 1
1. Plant tissue was prepared ( mustard and spinach). 2. The vegetable was dried in and oven of 110C Day 2 1. The dried vegetables was cut into pieces 2. About 3 grams of vegetable was weighed and placed in 250 ml beaker 3. 10 ml of nitric acid (HNO3) was added into the beaker and was allowed to stand Overnight
Day 3 A) Sample preparation 1. The sample was heated until red fume came out 2. Then it was cooled 3. 1 ml of peroxide (H2O2) was added into the cooled sample 4.The sample was reheated till concentrate 5. It was then filtered into 250ml volumetric flask 6. The sample was diluted with distilled water till marked B) Standard preparation Cr 1 ppm 2 ppm 3 ppm 4 ppm 5 ppm Volume 0.5 ml 1.0 ml 1.5 ml 2.0 ml 2.5 ml Cd 0.2 ppm 0.4 ppm 0.6 ppm 0.8 ppm 1.0 ppm Volume 1 ml 2 ml 3 ml 4 ml 5 ml
1. The standard was prepared as in table given above in 100 ml volumetric flask 2. The stock solution given was in 1000 ppm for each sample 3. 5 ml of the stock solution was pipette into a 100 ml volumetric flask and diluted with distilled water till marked 4. For each concentration, the volume was pipette into a 50 volumetric flask as stated in the table above respectively 5. 7 ml of the sample was pipette into each volumetric flask and was diluted with distiiled water till mark 6. The sample was then analyzed using atomic absorption spectroscopy
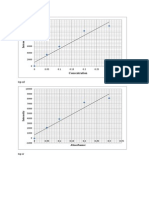
Results : CONCENTRATION CHROMIUM CADMIUM Blank Blank 1 ppm 0.2 ppm 2 ppm 0.4 ppm 3 ppm 0.6 ppm 4 ppm 0.8 ppm 5 ppm 1.0 ppm ABSORBANCE CHROMIUM CADMIUM 0.036 0.264 0.045 0.290 0.058 0.311 0.071 0.330 0.082 0.352 0.093 0.369 VOLUME CHROMIUM CADMIUM 0 0 0.5 ml 1 ml 1.0 ml 2 ml 1.5 ml 3 ml 2.0 ml 4 ml 2.5 ml 5 ml
Calculation : Least square method : CONCENTRATI ON Cr Cd Blank 1 ppm 2 ppm 3 ppm 4 ppm 5 ppm Blank 0.2 ppm 0.4 ppm 0.6 ppm 0.8 ppm 1.0 ppm ABSORBANCE (y1) Cr Cd 0.036 0.045 0.058 0.071 0.082 0.093 y= 0.385 0.264 0.290 0.311 0.330 0.352 0.369 y= 1.916 VOLUME (x1) Cr 0 0.5 ml 1.0 ml 1.5 ml 2.0 ml 2.5 ml
= 7.5
X1X1 Cr 0 0.25 1.00 2.25 4.00 6.25 xx= 13.75 Cd 0 1 4 9 16 25 xx= 55 Cr
X1Y1 Cd 0 0.290 0.622 0.990 1.408 1.845 xy= 5.155
Cd 0 1 ml 2 ml 3 ml 4 ml 5 ml
= 15
0 0.0225 0.0580 0.1065 0.1640 0.2325 xy= 0.5835
CHROMIUM
chromium
0.1 0.09 0.08 0.07 0.06 0.05 0.04 0.03 0.02 0.01 0 0 1 2 3 y = 0.0234x + 0.035 R = 0.9979
chromium Linear (chromium)
xy = ((xy) =0.5835 -
= 0.10225
xx = (xx)
= 13.75 = 4.375 m=
=
= 0.0234
= 0.035 Concentration of Chromium in plant tissue : C=
=
= 2.137 ppm
CADMIUM
cadmium
0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 1 2 3 4 5 6 cadmium Linear (cadmium) y = 0.0209x + 0.2672 R = 0.9965
xy = ((xy) =5.155 -
= 0.365
xx = (xx)
= 55 = 17.5 m=
=
= 0.0209
= 0.2671 Concentration of cadmium in plant tissue C=
=
=12.78 ppm
Discussions : In this experiment, we used wet digestion methods for sample preparation. It is for elemental analysis that involves the chemical degradation of sample matrices in solution, usually with a combination of acids to increase solubility as it has been done on day 2 and allow to stand overnight. The various acid and ux treatments are carry out at high temperatures in specially designed vessels that help to minimize contamination of the sample with substances in the air, the local environment, and from the vessel walls. Sample may be loss due to adsorption onto the vessel walls, volatilization, and coextraction, but these can be reduced by procedural modications. The use of closed systems, where the digestion reaction is completely isolated
from the surroundings, may help to reduce both contamination and sample loss. For this experiment, the sample was covered with aluminium foil. Standard addition method are particularly useful for analyzing complex samples in which the likelihood of matrix effects is substantial. A standard addition method can take several form. One of the most common form is spiking method as we had use it in preparing standard. Each solution was diluted to marked point before measuring or analyzing it. The least square method, is a typical calibration graph where I have plotted in calculation area for both sample chromium and cadmium respectively. The sample was injected into the atomic absorption spectroscopy and the result was obtained. Absorbance versus volume graph was plotted and the linear equation and R2 was calculated in Microsoft excel and as well as manually which had been shown. Conclusion : in this experiment, I had used mustard in determination of Chromium while for determination of Cadmium, I had used spinanch. I had achieved my objective to determine Cadmium and Chromium in plant tissue. The concentration of Cadmium in spinach is 12.78 ppm. The concentration of Chromium in mustard is 2.137 ppm.
References :
1. http://www.writescience.com/RMT%20PDFs/Elsevier/eans%20wetdig.pdf 2. http://www.newsmax.com/FastFeatures/chromium-health-benefitsnutrition/2011/01/21/id/369681 3. http://www.ipap.jp/proc/cs7/pdf/cs7_323.pdf 4. http://www.best-home-remedies.com/minerals/cadmium.htm 5. Principle of instrumental analysis 6th analysis, Douglas.A.Skoog.F.James Holler, Stanley R.Crouch
You might also like
- Chm580 Experiment 1Document9 pagesChm580 Experiment 1ohhiNo ratings yet
- Chm580 Experiment 2Document8 pagesChm580 Experiment 2ohhi100% (1)
- Lab Report Experiment 1 Chm624Document11 pagesLab Report Experiment 1 Chm624Hazwan HamimNo ratings yet
- Faculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Document7 pagesFaculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Husna Insyirah Bt SamadNo ratings yet
- Faculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Document6 pagesFaculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Husna Insyirah Bt SamadNo ratings yet
- CHM 580 Spectrochemical Methods of Analysis Laboratory Report Experiment 2: Ultraviolet-Visible SpectrosDocument10 pagesCHM 580 Spectrochemical Methods of Analysis Laboratory Report Experiment 2: Ultraviolet-Visible SpectrosAiNo ratings yet
- CHM 580 Exp 1 & 7Document19 pagesCHM 580 Exp 1 & 7Nabilah60% (5)
- Experiment 1 578Document12 pagesExperiment 1 578aisyah fauzi100% (1)
- Exp 4 chm556Document7 pagesExp 4 chm556Azli AzmanNo ratings yet
- Experiment 4 CHM 557 PDFDocument19 pagesExperiment 4 CHM 557 PDFinsyirah shazrinNo ratings yet
- Chm557 Laboratory Report: Experiment 5 The Robinson Annulation ReactionDocument14 pagesChm557 Laboratory Report: Experiment 5 The Robinson Annulation ReactionsyafNo ratings yet
- Sample Exp 6 CHM 477Document11 pagesSample Exp 6 CHM 477ommy madinaNo ratings yet
- Tutorial Chap 9 Ans-CHM580Document2 pagesTutorial Chap 9 Ans-CHM580tirahNo ratings yet
- CHM557 Exp 2Document12 pagesCHM557 Exp 2syafNo ratings yet
- CHM457 - Exp 4-Preparation of Aspirin - N.syamimi BT Zainal - As2452s1 PDFDocument5 pagesCHM457 - Exp 4-Preparation of Aspirin - N.syamimi BT Zainal - As2452s1 PDFsyamimi zainalNo ratings yet
- Lab Report CHM361Document6 pagesLab Report CHM361Nurin Izzati Zulkifli100% (1)
- Experiment 7 Chlorpyrifos chm510Document6 pagesExperiment 7 Chlorpyrifos chm510Amar SafwanNo ratings yet
- Experiment 2 HPLC chm510Document9 pagesExperiment 2 HPLC chm510Amar Safwan75% (12)
- CHM 510 Experiment 2Document16 pagesCHM 510 Experiment 2NabilahNo ratings yet
- Lab Report Experiment 5 Chm421Document9 pagesLab Report Experiment 5 Chm421aremyrah AzlanNo ratings yet
- Lab Report 7Document7 pagesLab Report 7Iena KasimNo ratings yet
- CHM557 Experiment 3 - Esterification Reaction of Vanillin - The Use of NMR To Determine A StructureDocument9 pagesCHM557 Experiment 3 - Esterification Reaction of Vanillin - The Use of NMR To Determine A StructureMamamiaNo ratings yet
- chm510 Experiment 3Document7 pageschm510 Experiment 3Naz Helmi100% (1)
- Experment 4 Analysis of Hydrocarbonsin Common Fuels Using Solid-Phase Microextraction (SPME) and Gas Chromatography-Mass Spectrometry (GC-MS)Document6 pagesExperment 4 Analysis of Hydrocarbonsin Common Fuels Using Solid-Phase Microextraction (SPME) and Gas Chromatography-Mass Spectrometry (GC-MS)NUR IZZATI OTHMAN BASRINo ratings yet
- CHM4201 Exp 8 - Gas LawDocument11 pagesCHM4201 Exp 8 - Gas Lawnajwa100% (2)
- Lab Report CHM 510Document30 pagesLab Report CHM 510Amirah AzlanNo ratings yet
- Spectrochemical Method of Analysis (CHM 580) EXPERIMENT 1:qualitative Analysis of Aspirin Phenacetin Caffeine and Sample Using FTIR and NMRDocument9 pagesSpectrochemical Method of Analysis (CHM 580) EXPERIMENT 1:qualitative Analysis of Aspirin Phenacetin Caffeine and Sample Using FTIR and NMRbatrisyia hazirahNo ratings yet
- Chm361 Lab ReportsDocument20 pagesChm361 Lab Reportswatiqah adilahNo ratings yet
- Esterification Reaction of Vanilin (The Use of Nuclear Magnetic Resonance and Infrared Spectroscopy To Determine The Structure)Document7 pagesEsterification Reaction of Vanilin (The Use of Nuclear Magnetic Resonance and Infrared Spectroscopy To Determine The Structure)Amirul Azhar88% (8)
- CHM 421 - Exp10Document4 pagesCHM 421 - Exp10AMIRAH ISHAMI ISHAKNo ratings yet
- Experiment 5 Analysis of Chlorpyrifos in Water Using Solid-Phase Extraction (SPE) and Gas Chromatography-Electron Capture Detector (GC-ECD)Document8 pagesExperiment 5 Analysis of Chlorpyrifos in Water Using Solid-Phase Extraction (SPE) and Gas Chromatography-Electron Capture Detector (GC-ECD)NUR IZZATI OTHMAN BASRINo ratings yet
- Frs 581Document7 pagesFrs 581Amy MusaNo ratings yet
- CHM361 Case Study PDFDocument1 pageCHM361 Case Study PDFHazwan0% (1)
- CHM457 Exp 6Document7 pagesCHM457 Exp 6Nur HismanizaNo ratings yet
- AbstractDocument5 pagesAbstractUnta Solehah67% (3)
- Exp 1 Chm510Document11 pagesExp 1 Chm510Nur Nadia Syahira100% (1)
- Suggested Answer - Tutorial 2 Chm510Document6 pagesSuggested Answer - Tutorial 2 Chm510Mark SullivanNo ratings yet
- chm421 Exp 3Document8 pageschm421 Exp 3Irfan Azahar100% (1)
- Laboratory Report CHM 457 Organic Chemistry: Universiti Teknologi Mara, Cawangan Perlis Kampus ArauDocument4 pagesLaboratory Report CHM 457 Organic Chemistry: Universiti Teknologi Mara, Cawangan Perlis Kampus ArauNasuha AriffinNo ratings yet
- Assignment Tokoh Organometallic GilmanDocument7 pagesAssignment Tokoh Organometallic GilmanniniNo ratings yet
- Sodium Boronhydride Reduction of CyclohexanoneDocument6 pagesSodium Boronhydride Reduction of CyclohexanoneWan Nur Amira91% (11)
- Exp5 520Document11 pagesExp5 520syamsaufi33% (3)
- Lab Report Experiment 3 4 and 5Document13 pagesLab Report Experiment 3 4 and 5Nurul Iman Che Awang90% (40)
- Experiment 3 CHM 457Document6 pagesExperiment 3 CHM 457Amirah Najihah100% (1)
- CHM 674 Exp 3Document10 pagesCHM 674 Exp 3nabilah hilmiNo ratings yet
- CHM557 Experiment 5 - The Robinson Annulation ReactionDocument9 pagesCHM557 Experiment 5 - The Robinson Annulation ReactionMamamia0% (1)
- Experiment 2: Analysis of An Unknown Vinegar SampleDocument7 pagesExperiment 2: Analysis of An Unknown Vinegar SampleNur Faizatul Atiqah100% (1)
- Experiment 3 CHM421Document8 pagesExperiment 3 CHM421pufff witches100% (1)
- CHM260 Experiment 4Document11 pagesCHM260 Experiment 4Muhammad Azri Haziq57% (7)
- Exp 3Document6 pagesExp 3ohhiNo ratings yet
- Lab 1 CHM 510 Complete 2011Document20 pagesLab 1 CHM 510 Complete 2011Nor Hasliza100% (1)
- CHM 361Document16 pagesCHM 361Siti Maizatul Akma100% (2)
- Experiment 5 Ink AnalysisDocument14 pagesExperiment 5 Ink AnalysisWanMohammadHajidiNo ratings yet
- Sodium Borohydride Reduction of CyclohexanoneDocument6 pagesSodium Borohydride Reduction of CyclohexanoneIqmal HakimiNo ratings yet
- CHM260 SWR Experiment 2Document6 pagesCHM260 SWR Experiment 2wnayNo ratings yet
- Preparation of 4-Methylcyclohexene: BackgroundDocument4 pagesPreparation of 4-Methylcyclohexene: BackgroundVanila AisNo ratings yet
- Experiment 2 CHM510Document24 pagesExperiment 2 CHM510Dang HumairahNo ratings yet
- Exp4 chm456Document8 pagesExp4 chm456Mawar AhmadNo ratings yet
- Atomic Absorption SpectrosDocument9 pagesAtomic Absorption Spectrosamirul azhar80% (10)
- Complete AAS N ICPDocument14 pagesComplete AAS N ICPMaxvicklye Rayner100% (1)
- Types of MicroorganismsDocument1 pageTypes of MicroorganismsAzreen AnisNo ratings yet
- Double Celebration Meal VoucherDocument1 pageDouble Celebration Meal VoucherAzreen AnisNo ratings yet
- Indices and Logarithm - ScribdDocument2 pagesIndices and Logarithm - ScribdAzreen AnisNo ratings yet
- SPM Form 4Document1 pageSPM Form 4Azreen AnisNo ratings yet
- Kedah Module Peningkatan Prestasi Tingkatan 5 SPM 2014 English (D1FA424A)Document31 pagesKedah Module Peningkatan Prestasi Tingkatan 5 SPM 2014 English (D1FA424A)Azreen Anis100% (1)
- English Year 3 Paper 2 140204012751 Phpapp01Document6 pagesEnglish Year 3 Paper 2 140204012751 Phpapp01Zatul Izawati Ahmad IbrahimNo ratings yet
- Vision Note For Malaysia SPM Form 4Document1 pageVision Note For Malaysia SPM Form 4Azreen AnisNo ratings yet
- Fs 11900Document7 pagesFs 11900Azreen AnisNo ratings yet
- Tution NsDocument2 pagesTution NsAzreen AnisNo ratings yet
- BOD ProcedureDocument14 pagesBOD ProcedureSajith Ranatunga100% (1)
- BOD ProcedureDocument14 pagesBOD ProcedureSajith Ranatunga100% (1)
- Img 0003Document1 pageImg 0003Azreen AnisNo ratings yet
- + y 9. Give Your Answer To Two DecimalDocument4 pages+ y 9. Give Your Answer To Two DecimalAzreen AnisNo ratings yet
- Shafiq Add Math Test 1Document5 pagesShafiq Add Math Test 1Azreen AnisNo ratings yet
- AtrDocument4 pagesAtrAzreen AnisNo ratings yet
- Trial Kedah English SPM 2013 k2 Dan JawapanDocument25 pagesTrial Kedah English SPM 2013 k2 Dan Jawapancmfoo2988No ratings yet
- Time Table Test Session Mac - JUN 2012 Date Time Course Code Test PlaceDocument1 pageTime Table Test Session Mac - JUN 2012 Date Time Course Code Test PlaceAzreen AnisNo ratings yet
- Rate Laws and StoichiometryDocument20 pagesRate Laws and StoichiometryAzreen AnisNo ratings yet
- Tution NsDocument2 pagesTution NsAzreen AnisNo ratings yet
- Icp CDDocument1 pageIcp CDAzreen AnisNo ratings yet
- Universiti Teknologi Mara Final Examination: Confidential AS/APR 2006/CHWI561Document3 pagesUniversiti Teknologi Mara Final Examination: Confidential AS/APR 2006/CHWI561Azreen AnisNo ratings yet
- Tution NsDocument2 pagesTution NsAzreen AnisNo ratings yet
- April 2011Document4 pagesApril 2011Azreen AnisNo ratings yet
- Analytical Quality Assurance - Who PDFDocument17 pagesAnalytical Quality Assurance - Who PDFvabimhahNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Oct 2007Document4 pagesOct 2007Azreen AnisNo ratings yet
- CHM580Document7 pagesCHM580Azreen AnisNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Nota PendekDocument9 pagesNota PendekBeevy GB71% (7)
- Paper 2 There Are Three Questions in This Paper. Answer All The Questions. Section ADocument7 pagesPaper 2 There Are Three Questions in This Paper. Answer All The Questions. Section AAzreen AnisNo ratings yet
- DigfiltDocument237 pagesDigfiltJuhi SinghNo ratings yet
- Trigonometric Substitution: Jzfa20Document12 pagesTrigonometric Substitution: Jzfa20Samuel SmallmanNo ratings yet
- ZB Scroll Compressors ManualDocument70 pagesZB Scroll Compressors ManualJavier AffifNo ratings yet
- Craig Pirrong-Commodity Price Dynamics - A Structural Approach-Cambridge University Press (2011) PDFDocument239 pagesCraig Pirrong-Commodity Price Dynamics - A Structural Approach-Cambridge University Press (2011) PDFchengadNo ratings yet
- Prime Number FactorizationDocument10 pagesPrime Number FactorizationedithaenriquezNo ratings yet
- WSU Presentation 20220407Document63 pagesWSU Presentation 20220407debapriyoNo ratings yet
- Builders' Metalwork: Ci/Sfb 21.9 Xt6Document32 pagesBuilders' Metalwork: Ci/Sfb 21.9 Xt6JC TsuiNo ratings yet
- Calibration of The Continuous Surface Cap Model For ConcreteDocument19 pagesCalibration of The Continuous Surface Cap Model For ConcreteAbhijit KulkarniNo ratings yet
- Online Food Ordering System MiniDocument11 pagesOnline Food Ordering System Minijwala reddy83% (47)
- How Do Bodies MoveDocument28 pagesHow Do Bodies Movedeme2411No ratings yet
- Chameleon ChipDocument2 pagesChameleon ChipChetan KumarNo ratings yet
- Electricity Markets PDFDocument2 pagesElectricity Markets PDFAhmed KhairiNo ratings yet
- AlternatorDocument14 pagesAlternatorTaraknath MukherjeeNo ratings yet
- L071ME4182DFADocument81 pagesL071ME4182DFAmegamech23No ratings yet
- Visvesvaraya Technological University: "Dijkstra'S Algorithm"Document34 pagesVisvesvaraya Technological University: "Dijkstra'S Algorithm"RASHMINo ratings yet
- Tutorial Book 0.4Document27 pagesTutorial Book 0.4TonyandAnthonyNo ratings yet
- Investment Model QuestionsDocument12 pagesInvestment Model Questionssamuel debebe0% (1)
- 50 Circular 2022Document1 page50 Circular 2022Shaurya BansalNo ratings yet
- Data Sheet - enDocument2 pagesData Sheet - enrodriggoguedesNo ratings yet
- TM 9-4110-241-23PDocument41 pagesTM 9-4110-241-23PwwwsurvivalebookscomNo ratings yet
- Shear Force & Bending Moment TestDocument11 pagesShear Force & Bending Moment TestKalaiArasanNo ratings yet
- Problem in Traffic Flow Theory: Speed-Density Relationships: Lnu LNK Q U KDocument10 pagesProblem in Traffic Flow Theory: Speed-Density Relationships: Lnu LNK Q U KRomel DecenillaNo ratings yet
- Bogiflex KGD20 - For PlantDocument13 pagesBogiflex KGD20 - For PlantAnonymous PVXBGg9TNo ratings yet
- 4333105.56 Ledenvo Led ST 60w 757 Vs1 Osram-TrfDocument12 pages4333105.56 Ledenvo Led ST 60w 757 Vs1 Osram-TrfFathulNo ratings yet
- Allen-Bradley RSLogix 500 PDFDocument6 pagesAllen-Bradley RSLogix 500 PDFGanda PutraNo ratings yet
- West Knits Book 3Document56 pagesWest Knits Book 3Alexandr Maxiuta100% (17)
- Grade9 Physics PDFDocument2 pagesGrade9 Physics PDFRajNo ratings yet
- SIF Corporate-Presentatie 2017Document35 pagesSIF Corporate-Presentatie 201766apenlullenNo ratings yet
- Owners Manual 2018Document49 pagesOwners Manual 2018Marv-Vic SantosNo ratings yet