Professional Documents
Culture Documents
Fundamentals of Reaction Kinetics
Uploaded by
chantaiahOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fundamentals of Reaction Kinetics
Uploaded by
chantaiahCopyright:
Available Formats
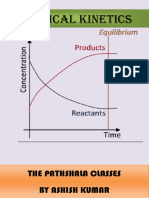
1 FUNDAMENTALS OF REACTION KINETICS Introduction The branch of Physical Chemistry which deals with the 'speed' or 'rate' at which
a reaction occurs is called Chemical Kinetics. The study of chemical kinetics, therefore, includes the rate of a chemical reaction as also the 'factors' which influence its rate. The Significance of study of kinetics of reactions is two-fold. It provides very useful information on how chemical reactions occur or what is their mechanism. Also, the knowledge of the rate of reactions is very valuable for the success of an industrial process Where it is imperative to select optimum conditions of the reactions involved when these reactions proceed at a rate so as to give maximum yield. RATE OF A REACTION Rate of a reaction refers to the change in concentration of reactants and the products with respect to time as the chemical reaction proceeds. Rate of a reaction can be expressed in two ways: qualitatively and quantitatively. By using in exact words such as 'slow' or 'fast' we simply express the rate of reactions qualitatively. For instance, When Hcl is added to Zn pieces instantaneously H2 gas is evolved so this reaction can be said very fast reaction. On the other hand A piece of iron may be rusted in several months or years even and may be termed as 'very slow'. Definition Rate of a reaction can be defined as the decrease in concentration of reactants or increase in concentration of products with respect to time. Mathematically it can be expressed as shown below: Rate of a reaction = Change in concentration of reactants or products Time
2 If concentration is expressed by x then the change in concentration is expressed by dx Then dx is positive for the products since the concentration of products increases with time, where as for reactants dx is negative since the concentration of reactants decrease with time. Then the rate of reaction can also be expressed as Rate = dx dT
Where dx --- the change in concentration of products dT--- the change in time Or Rate = -dx dT
Where dx --- the change in concentration of reactants dT--- the change in time
FACTORS AFFECTING THE REACTlON RATE Several factors influence the rate of a Chemical reaction. Significant of these are discussed below: (1) Nature of Reactants: Reaction between polar or ionic molecules is very fast and occurs almost instantaneously. On the other hand, reactions in which bonds are arranged or electrons are transferred take longer time than ionic reactions. (2) Effect of Concentration: The concentration of reactants and products has profound effect on the rate of reaction. According to collision theory the number of collisions between the reacting molecules increases if concentration of reactants is increased leading to increase of rate of reaction. Thus an increase in the concentration of reactants increases the rate of reaction.
3 (3) Effect of Temperature: The rate of a reaction is increased considerably by a little increase in temperature. The effect of temperature on the rate of the reaction can be explained by considering the Arrehenious rate equation which is given below Rate = A e-Q/RT Where A---Pre exponential constant Q---Activation energy R---Universal gas constant T---Temperature in K From the above equation we can conclude that the rate of a reaction increases exponentially with temperature. Molecularity of a reaction The Molecularity is the number of reacting molecules which are participated in the chemical reaction irrespective of the change in their concentration. Example: A + B = C + D The Molecularity of the above equation is two as the no. of molecules participated in the reaction is two (that is one molecule of A and one molecule of B) Order of a reaction The Order of a reaction is the number of reacting molecules which are actively participated in the chemical reaction and whose concentration under goes a change. Example: A + B = C + D If the rate of the above equation is expressed as dC/dT = K [A] [B] Then the order is --1+1 = 2 In general: mA + nB = oP + qR Rate = dC/dT = K [A]m[B]n Then the order of the reaction is -- m+n K --- the rate constant.
4 FIRST ORDER REACTION In a reaction of the first order, only one molecule is necessary for the reaction to proceed and the change may be represented in a general way as:
PRODUCTS
Let a be the initial concentration of A in gm. moles litre-l and suppose that after an interval of time t, the concentration is (a-x) gm. moles litre-l. The rate of the above reaction is given by the expression: dx dT Where k is known as rate constant. dx (a-x) Or -------------------------------= k dt (1) = k (a-x)
Integration of the expression (1) gives Or dx (a-x) = k dt
Or
-ln (a-x) = kt +I
--------------------------------(2)
Where I is the constant of integration. The constant k may be evaluated by putting t=0 and x = 0. Thus I = -ln a Substituting for I in equation (2) ln a a-x 1 t a a-x Or k= =kt
ln
The above equation is called rate equation of the first order reaction. The value of k can be found by substituting the values of a and (a - x) determined experimentally at time interval t during the course of the reaction.
Examples of first order reactions Some common reactions which follow first order kinetics are listed below: (1) Decomposition of N2O5sin CCl4soIution Nitrogen pentoxide in carbon tetrachloride solution decomposes to form oxygen gas, N2O5 2NO2 + O2 (2) Decomposition of H202 in aqueous solution. The decomposition of H202in the presence of Pt as catalyst is a first order reaction. H202 (3) All radio active decay reactions SECOND ORDER REACTION The second order reaction can be taken as shown below 2A PRODUCTS
H2O + O
Suppose the initial concentration of A is a moleslitre-1 if after time t, x moles of A have reacted, the concentration of A is (a- x).We know that for such a second order reaction, rate of reaction is proportional to the square of the concentration of the reactant. Thus, dx dt = k(a-x)2
---------------------------------(1)
Where k is the rate constant Rearranging equation (1), we have
--------------------------------(2)
dx (a-x)2
k dt
On integration, it gives -----------------------------(3)
1 (a-x)
kt + I
Where I is integration constant I can be evaluated by putting x =0 and t = 0. Thus,
----------------------------------(4) Substituting for I in equation (3)
1 a
1 (a-x)
kt + 1/a
Thus k =
1 t
x a(a-x)
The above equation is called rate equation of a second order reaction. Examples of second order reactions 1) Hydrolysis of an Ester by NaOH. This is a typical second order reaction. CH3COOC2H5 (ethyl acetate) + NaOH CH3COONa + C2H5OH (ethyl alcohol)
DETERMINATION OF ORDER OF A REACTION BY HALF LIFE METHOD Two separate experiments are performed by taking different initial concentrations of a reactant. The progress of the reaction in each case is recorded by analysis. When the initial concentration is reduced to one-half, the time is noted. Let the initial concentrations in the two experiments be [a1] and [a2], while times for completion of half change are t1 and t2 respectively.
7 We know that half-life period for a first order reaction is independent of the initial concentration, a We also know: Half - life 1 a
For 2nd order reaction
Half - life
1 a2
For 3rd order reaction
Half - life
1 an-1
For nth order reaction
Substituting values of initial concentrations and half-life periods from the two experiments, we have
1 a1n-1
t1
1 a2n-1 and t2 t1 ln [ a2 a1 =[ a1 a2
t2
n-1
(n-1)
= Ln[
t1 t2
Solving for n, the order of reaction n = 1 + ln[t1/t2] ln[a2/a1]
You might also like
- Exercise 8 Kinetics of Hydrolysis of Ethyl AcetateDocument6 pagesExercise 8 Kinetics of Hydrolysis of Ethyl AcetatePalak BansalNo ratings yet
- Reaction MechanismsDocument17 pagesReaction MechanismskimNo ratings yet
- Order of ReactionDocument11 pagesOrder of ReactionBadar RizwanNo ratings yet
- Chem Chapt13 PractiseDocument5 pagesChem Chapt13 PractiseqwerNo ratings yet
- Unit-I 10Document17 pagesUnit-I 10Dude BoysNo ratings yet
- Chemistry Form 6 Chap 05 NewDocument83 pagesChemistry Form 6 Chap 05 Newmusafir24No ratings yet
- Orderreactions 140122012437 Phpapp01Document47 pagesOrderreactions 140122012437 Phpapp01kunalkushwah4141No ratings yet
- Lecture 1425072667Document35 pagesLecture 1425072667Mahesh Babu TalupulaNo ratings yet
- Xii - CH4 - Chemical KineticsDocument3 pagesXii - CH4 - Chemical KineticsYash RajNo ratings yet
- Chemistry Pre-U Chemistry Sem 1 Chap 5 PDFDocument85 pagesChemistry Pre-U Chemistry Sem 1 Chap 5 PDFJIANHUI0160% (1)
- Chemical Kinetics Review 2013Document4 pagesChemical Kinetics Review 2013anonymoose9No ratings yet
- Unit-7 Chemical Kinetics 2023Document13 pagesUnit-7 Chemical Kinetics 2023jagannathanNo ratings yet
- Chemical KineticsDocument11 pagesChemical KineticsSrijan GoyalNo ratings yet
- Chemical KinaticsDocument22 pagesChemical KinaticsAsif SiamNo ratings yet
- Document From JenDocument51 pagesDocument From JenAksh GuptaNo ratings yet
- Chemical KineticsDocument15 pagesChemical KineticsNikhil AgarwalNo ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsManohar MaripeNo ratings yet
- Chemistry Unit 5.4Document8 pagesChemistry Unit 5.4Sonal PereraNo ratings yet
- Chemical Kinetics Type 1Document32 pagesChemical Kinetics Type 1DeependraNo ratings yet
- CHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Document8 pagesCHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Vijay Kumar100% (1)
- Content Marketed & Distributed By: Chemical KineticsDocument9 pagesContent Marketed & Distributed By: Chemical KineticsPrithviraj NetkeNo ratings yet
- Rate of ReactionDocument44 pagesRate of ReactionFitsum DemissieNo ratings yet
- Reaction Rates, GE 404Document6 pagesReaction Rates, GE 404mevadatinkalNo ratings yet
- Chemical KineticsDocument134 pagesChemical Kineticstapas kundu100% (1)
- CH 7 - Chemical KineticsDocument60 pagesCH 7 - Chemical KineticsCharbel RahmeNo ratings yet
- DP Chemical KineticsDocument32 pagesDP Chemical KineticsAniket RayNo ratings yet
- Notes Chemical KineticsDocument17 pagesNotes Chemical KineticsAMAR KUMARNo ratings yet
- Chemistry Unit 4Document62 pagesChemistry Unit 4YoNo ratings yet
- Chemical Kinetics-MEDocument22 pagesChemical Kinetics-MEprishaNo ratings yet
- KineticsDocument51 pagesKineticsSaumil Sachdeva100% (1)
- Reaction KineticsDocument37 pagesReaction KineticsDaisyNo ratings yet
- Che Kine2Document229 pagesChe Kine2sivaram888No ratings yet
- KineticsDocument26 pagesKineticsMelissa M. Abansi-BautistaNo ratings yet
- Topic 4.1 Kinetics Rate Equations Determining Orders of Reaction Explaining Orders of Reaction Effect of Changing Conditions On The Rate ConstantDocument9 pagesTopic 4.1 Kinetics Rate Equations Determining Orders of Reaction Explaining Orders of Reaction Effect of Changing Conditions On The Rate ConstantxcomNo ratings yet
- Reaction Rate: A+B ABDocument5 pagesReaction Rate: A+B ABFaisal Mohad Al SakhenNo ratings yet
- Chapter 14Document42 pagesChapter 14Dana CapbunNo ratings yet
- Orderofareaction2302 151029150927 Lva1 App6892Document25 pagesOrderofareaction2302 151029150927 Lva1 App6892kunalkushwah4141No ratings yet
- Chemical KineticsDocument41 pagesChemical Kineticskishangopi1230% (1)
- Chemical Kinetics TheoryDocument30 pagesChemical Kinetics TheoryBichitra GautamNo ratings yet
- Chemical Kinetics PDFDocument9 pagesChemical Kinetics PDFPriyanshu amanNo ratings yet
- Explain Reaction MechanismDocument5 pagesExplain Reaction MechanismQuynh NhuNo ratings yet
- Chemical and Enzyme Kinetics Lecture 2Document47 pagesChemical and Enzyme Kinetics Lecture 2downdstairs45No ratings yet
- Chemical Kinetics Part IDocument45 pagesChemical Kinetics Part IKarthikanAmirthalingamNo ratings yet
- Revision Notes For Class 12 CBSE Chemistry, Chemical Kinetics - TopperlearningDocument5 pagesRevision Notes For Class 12 CBSE Chemistry, Chemical Kinetics - TopperlearningRishabh Bhandari0% (1)
- Chemical KineticsDocument10 pagesChemical KineticsTanayaa PatilNo ratings yet
- One Stop For Colleges Education Career: Minglebox Engineering PrepDocument14 pagesOne Stop For Colleges Education Career: Minglebox Engineering PrepVigneshwar DhamodharanNo ratings yet
- A2 Chemistry Unit 4 NotesDocument27 pagesA2 Chemistry Unit 4 NotesRebecca78% (9)
- Chemical KineticsDocument72 pagesChemical KineticsSiddhartha KumarNo ratings yet
- AP Chemistry Notes: Chapter 12 Chemical Kinetics: 12.1 Reaction RatesDocument10 pagesAP Chemistry Notes: Chapter 12 Chemical Kinetics: 12.1 Reaction RatesAhsen BilalNo ratings yet
- Topic 4.1 Kinetics Rate Equations Determining Orders of Reaction Explaining Orders of Reaction Effect of Changing Conditions On The Rate ConstantDocument10 pagesTopic 4.1 Kinetics Rate Equations Determining Orders of Reaction Explaining Orders of Reaction Effect of Changing Conditions On The Rate ConstantNur Kintan ApriliaNo ratings yet
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Barron's Physics Practice Plus: 400+ Online Questions and Quick Study ReviewFrom EverandBarron's Physics Practice Plus: 400+ Online Questions and Quick Study ReviewNo ratings yet
- 74Document1 page74chantaiahNo ratings yet
- Tips and TricksDocument1 pageTips and TrickschantaiahNo ratings yet
- Arch Book Solution Ch12 SepDocument30 pagesArch Book Solution Ch12 SepchantaiahNo ratings yet
- Adj ListDocument1 pageAdj ListchantaiahNo ratings yet
- DS GFGDocument5 pagesDS GFGchantaiahNo ratings yet
- IDs of IOI ACMDocument4 pagesIDs of IOI ACMchantaiahNo ratings yet
- Arch Book Solution Ch7 SepDocument17 pagesArch Book Solution Ch7 SepchantaiahNo ratings yet
- 03 04 09Document12 pages03 04 09chantaiah100% (1)
- Arch Book Solution Ch15 SepDocument17 pagesArch Book Solution Ch15 SepchantaiahNo ratings yet
- Arch Book Solution Ch7 SepDocument17 pagesArch Book Solution Ch7 SepchantaiahNo ratings yet
- Arch Book Solution Ch10 SepDocument20 pagesArch Book Solution Ch10 SepchantaiahNo ratings yet
- Arch Book Solution Ch2 SepDocument37 pagesArch Book Solution Ch2 SepchantaiahNo ratings yet
- Arch Book Solution Ch10 SepDocument20 pagesArch Book Solution Ch10 SepchantaiahNo ratings yet
- Arch Book Solution Ch9 SepDocument35 pagesArch Book Solution Ch9 SepchantaiahNo ratings yet
- Arch Book Solution Ch4 SepDocument29 pagesArch Book Solution Ch4 SepchantaiahNo ratings yet
- Arch Book Solution Ch5 SepDocument27 pagesArch Book Solution Ch5 SepchantaiahNo ratings yet
- Ioi and Acm GroupDocument4 pagesIoi and Acm GroupchantaiahNo ratings yet
- Dual 4-Input Multiplexer SN54/74LS153: Low Power SchottkyDocument3 pagesDual 4-Input Multiplexer SN54/74LS153: Low Power SchottkyAriel HdezNo ratings yet
- Operational Amplifier BasicsDocument6 pagesOperational Amplifier BasicschantaiahNo ratings yet
- DS GFGDocument5 pagesDS GFGchantaiahNo ratings yet
- Arch Book Solution Ch3 SepDocument32 pagesArch Book Solution Ch3 SepchantaiahNo ratings yet
- Data SheetsDocument3 pagesData SheetschantaiahNo ratings yet
- New Text On Computer ArchitechtureDocument20 pagesNew Text On Computer ArchitechturechantaiahNo ratings yet
- Registration Guidelines 2014 December SessionDocument1 pageRegistration Guidelines 2014 December SessionchantaiahNo ratings yet
- T1-842-2014-Dts-Proceedings - 2 - Dte - 207.Document1 pageT1-842-2014-Dts-Proceedings - 2 - Dte - 207.chantaiahNo ratings yet
- Undertaking For PicnicDocument3 pagesUndertaking For PicnicchantaiahNo ratings yet
- Dual 4-Input Multiplexer SN54/74LS153: Low Power SchottkyDocument3 pagesDual 4-Input Multiplexer SN54/74LS153: Low Power SchottkyAriel HdezNo ratings yet
- 74LS90 Contador AsincronoDocument7 pages74LS90 Contador AsincronoFISRAELGRNo ratings yet
- Gooby HTMLDocument6 pagesGooby HTMLchantaiahNo ratings yet
- Hello Words in French Revolution in My Mind BitsessssssssssssssssssssssssssssssssDocument64 pagesHello Words in French Revolution in My Mind BitsessssssssssssssssssssssssssssssssMax Vallejo FactorNo ratings yet