Professional Documents
Culture Documents
Organometal - PDF JFNGFGJFNGV FJGFJGJNG FJGNRNGJN Dfdjje
Organometal - PDF JFNGFGJFNGV FJGFJGJNG FJGNRNGJN Dfdjje
Uploaded by
rvignesh2809Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organometal - PDF JFNGFGJFNGV FJGFJGJNG FJGNRNGJN Dfdjje
Organometal - PDF JFNGFGJFNGV FJGFJGJNG FJGNRNGJN Dfdjje
Uploaded by
rvignesh2809Copyright:
Available Formats
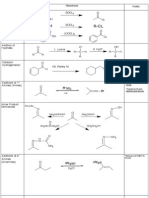
Alkyl Halides Organometallic Reagents 1) Grignard R X + Mg0
ether or THF
R MgII X now nucleophilic
reacts with: H+, D+ electrophile (H2O, D2O) O R R or R O H
HO
1 2 3
6 5 4 1 2
HO
3
6 5 4
disconnect between carbons 2 and 3
MgBr
O +
6 3 4 5
MgBr O
1 2 3 6 5 4
H2O
HO
1 2 3
6 5 4
2) Organolithium a) Li n-butyl lithium H3C H3C
b)
2 H3C
Li + CuI
ether
CuLi +
LiI
Formation
H3C H3C
CuLi
2 1
ether
1
LiI
CH3Cu Reaction
any alkyl group
Remember pKa of alkyl group is ~50 (very high) so deprotonated alkyl group is VERY basic
H3C CH2 H2 H3C C Li
NaBr
very basic carbons
will react w/ acidic proton like H2O (pKa 15.7) HC CH (pKa 25)
Not mentioned in class: Chapter 10.10 Be familiar with terminology, oxidation, and reduction. In inorganic/general chemistry: (M=metal) M0 M2+ Organic chemistry: reduction: gain in electron density by carbon oxidation: loss of electron density by carbon In practice: oxidation reduction C O C N C X bonds will form M2+ oxidation M0 reduction
C H break C H will form C O A very common example: H2C CH2 Pd/C N2 H3C CH3 Reduction C N C X bonds will break
You might also like
- Summary of Important Organic ReactionsDocument41 pagesSummary of Important Organic ReactionsKathyNo ratings yet
- Orgo Reaction SheetDocument9 pagesOrgo Reaction SheetKyle Broflovski100% (1)
- Alcohol SummaryDocument3 pagesAlcohol SummarydanielmahsaNo ratings yet
- PDFDocument12 pagesPDFJhonsonNo ratings yet
- 03 Hydro (Alkanes Theory 01)Document16 pages03 Hydro (Alkanes Theory 01)ayushNo ratings yet
- Alcohol, Phenols and Ethers Ch-10Document19 pagesAlcohol, Phenols and Ethers Ch-10Literal ShTNo ratings yet
- Chapter 12 Study Guide PDFDocument44 pagesChapter 12 Study Guide PDFkNo ratings yet
- Organic Chemistry 4 Edition: Reactions of Alcohols, Ethers, Epoxides, and Sulfur-Containing CompoundsDocument50 pagesOrganic Chemistry 4 Edition: Reactions of Alcohols, Ethers, Epoxides, and Sulfur-Containing CompoundsDella AinurrohmaNo ratings yet
- 12 Chemistry Keypoints Revision Questions Chapter 12Document20 pages12 Chemistry Keypoints Revision Questions Chapter 12sangam patraNo ratings yet
- Hydrocarbons.Document70 pagesHydrocarbons.Mandar Sheth100% (1)
- Chapter 14Document18 pagesChapter 14haterNo ratings yet
- Halogen+Compound+ +Ex+I+by+PC+SirDocument8 pagesHalogen+Compound+ +Ex+I+by+PC+SirSuraj SinghNo ratings yet
- Hydrocarbon LatestDocument23 pagesHydrocarbon LatestHimanshu100% (1)
- Alkyl Halide and Aryl HalideDocument46 pagesAlkyl Halide and Aryl Halidekartikbarot884No ratings yet
- Haloalkanes and HaloarenesDocument14 pagesHaloalkanes and HaloarenesKalpesh BishnoiNo ratings yet
- Alcohol Phenol Ether-Part IIDocument4 pagesAlcohol Phenol Ether-Part IIkakoliNo ratings yet
- Alkil HalidaDocument56 pagesAlkil HalidaZikriDWafiNo ratings yet
- CLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Document36 pagesCLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Utkarsh KumarNo ratings yet
- AlcoholsDocument16 pagesAlcoholsNirvaniNo ratings yet
- Flow Charts in Organic ChemistryDocument16 pagesFlow Charts in Organic ChemistryJessie McCartney85% (27)
- Organic Chemistry MinDocument18 pagesOrganic Chemistry Mina711789322No ratings yet
- Alcohols 2Document15 pagesAlcohols 2Junaid KhanNo ratings yet
- H2 Chemistry (9729) Lecture Notes 13 - Organic Chemistry Halogen DerivativesDocument27 pagesH2 Chemistry (9729) Lecture Notes 13 - Organic Chemistry Halogen DerivativesArvin LiangdyNo ratings yet
- Chapter 8 Reactions of AlcoholsDocument12 pagesChapter 8 Reactions of AlcoholsRoberto SIlvaNo ratings yet
- Presentation Alkyl HalidesDocument20 pagesPresentation Alkyl HalidesCaroline MuthoniNo ratings yet
- CH 17Document18 pagesCH 17MirjanaNo ratings yet
- Haloalkanes & HaloarenesDocument10 pagesHaloalkanes & Haloarenesakshatshukla2021No ratings yet
- OC - Halogen Derivatives - EDocument100 pagesOC - Halogen Derivatives - EJohn Doe100% (1)
- Haloalkanes and HaloarenesDocument14 pagesHaloalkanes and Haloarenesshreyansh tanwarNo ratings yet
- 12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Document47 pages12 Chemistry Impq CH10 Haloalkanes and Haloarenes 02Swaroop SurendraNo ratings yet
- Chapter 1 ALCOHOLDocument65 pagesChapter 1 ALCOHOLNURUL AINUN MUHAMMAD NOR100% (1)
- CHEM 215 F12 Chapter 13 Notes UMICHDocument13 pagesCHEM 215 F12 Chapter 13 Notes UMICHRoxanne IlaganNo ratings yet
- The University of Zambia School of Natural Sciences: Chemistry DepartmentDocument48 pagesThe University of Zambia School of Natural Sciences: Chemistry Departmentmartin mulengaNo ratings yet
- 16 Hydroxyl compound-24-STDDocument38 pages16 Hydroxyl compound-24-STDManh Doan DucNo ratings yet
- Oxygen Containing CompoundsDocument27 pagesOxygen Containing CompoundsSHIVI DwivediNo ratings yet
- Alcohals Phenols AsDocument44 pagesAlcohals Phenols AsAmit RoutNo ratings yet
- 03 Alkyl Halides - (Theory-01)Document28 pages03 Alkyl Halides - (Theory-01)ishaant chauhanNo ratings yet
- Alkyl HalidesDocument54 pagesAlkyl HalidesSaurabh KumarNo ratings yet
- Chemistry: AlcoholsDocument11 pagesChemistry: AlcoholsGaelle TomkoNo ratings yet
- L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.1Document21 pagesL.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.1IEyra ShaHeraNo ratings yet
- Dhoom # 9 Haloalkane & Haloarene in One Shot (10.6.2020)Document156 pagesDhoom # 9 Haloalkane & Haloarene in One Shot (10.6.2020)Jeet RathodNo ratings yet
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- Raffles Junior College JC2 H2 Chemistry 2007/8 Suggested Answers To Nov 2007 Chemistry 9746 Paper 1Document16 pagesRaffles Junior College JC2 H2 Chemistry 2007/8 Suggested Answers To Nov 2007 Chemistry 9746 Paper 1Ah XiuNo ratings yet
- Class - 12/chemistry-2 - Reactions of AlcoholsDocument60 pagesClass - 12/chemistry-2 - Reactions of AlcoholsAISHA AHAMMEDNo ratings yet
- Aldehydes & Ketones (Additional)Document24 pagesAldehydes & Ketones (Additional)Michael Angelo FilomenoNo ratings yet
- Alkyl HalidesDocument16 pagesAlkyl HalidesGmd MrloNo ratings yet
- HaloalkeneDocument20 pagesHaloalkeneRashmi GuptaNo ratings yet
- CBSE Class 12 Chem Notes Question Bank Alcohols Phenols and Ethers PDFDocument19 pagesCBSE Class 12 Chem Notes Question Bank Alcohols Phenols and Ethers PDFParam MNo ratings yet
- Halogens Power Point NotesDocument74 pagesHalogens Power Point NotesgajenraoNo ratings yet
- Ether (Theory) Module-4Document7 pagesEther (Theory) Module-4Raju SinghNo ratings yet
- 45 Hydrocarbons AlkanesDocument7 pages45 Hydrocarbons Alkanessujalgupta0123456789No ratings yet
- 7.0 Haloalkanes 20212022Document88 pages7.0 Haloalkanes 20212022ZULAIKA BINTI SALLEH A22BE0415No ratings yet
- Carbonyl Compounds Aldehydes and KetonesDocument62 pagesCarbonyl Compounds Aldehydes and KetonesSubhabrata MabhaiNo ratings yet
- Hydroxy Compounds: (Alcohols)Document71 pagesHydroxy Compounds: (Alcohols)NorsyazaEdmiraNo ratings yet
- Substitution Elim HandoutsDocument8 pagesSubstitution Elim HandoutsT Smith AndresNo ratings yet
- Exercise 1 1683183099Document27 pagesExercise 1 1683183099shivam126921No ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- IDs of IOI ACMDocument4 pagesIDs of IOI ACMchantaiahNo ratings yet
- DS GFGDocument5 pagesDS GFGchantaiahNo ratings yet
- Registration Guidelines 2014 December SessionDocument1 pageRegistration Guidelines 2014 December SessionchantaiahNo ratings yet
- Undertaking For PicnicDocument3 pagesUndertaking For PicnicchantaiahNo ratings yet
- WWW Codechef Com DEC14 Problems CHEFBRDocument3 pagesWWW Codechef Com DEC14 Problems CHEFBRchantaiahNo ratings yet